Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist
- PMID: 23089394
- PMCID: PMC3504137
- DOI: 10.4049/jimmunol.1202339
Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist
Abstract
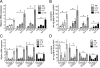
When excessively activated or deregulated, complement becomes a major link between infection and inflammatory pathology including periodontitis. This oral inflammatory disease is associated with a dysbiotic microbiota, leads to the destruction of bone and other tooth-supporting structures, and exerts an adverse impact on systemic health. We have previously shown that mice deficient either in complement C5a receptor (C5aR; CD88) or TLR2 are highly and similarly resistant to periodontitis, suggesting that a cross-talk between the two receptors may be involved in the disease process. In this paper, we show that C5aR and TLR2 indeed synergize for maximal inflammatory responses in the periodontal tissue and uncover a novel pharmacological target to abrogate periodontitis. Using two different mouse models of periodontitis, we show that local treatments with a C5aR antagonist inhibited periodontal inflammation through downregulation of TNF, IL-1β, IL-6, and IL-17 and further protected against bone loss, regardless of the presence of TLR2. These findings not only reveal a crucial cooperation between C5aR and TLR2 in periodontal inflammation but also provide proof-of-concept for local targeting of C5aR as a powerful candidate for the treatment of human periodontitis.
Figures
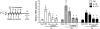
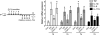



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

