Type I interferon signaling protects mice from lethal henipavirus infection
- PMID: 23089589
- PMCID: PMC7107294
- DOI: 10.1093/infdis/jis653
Type I interferon signaling protects mice from lethal henipavirus infection
Abstract
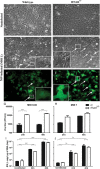
Hendra virus (HeV) and Nipah virus (NiV) are closely related, recently emerged paramyxoviruses that form Henipavirus genus and are capable of causing considerable morbidity and mortality in a number of mammalian species, including humans. However, in contrast to many other species and despite expression of functional virus entry receptors, mice are resistant to henipavirus infection. We report here the susceptibility of mice deleted for the type I interferon receptor (IFNAR-KO) to both HeV and NiV. Intraperitoneally infected mice developed fatal encephalitis, with pathology and immunohistochemical features similar to what was found in humans. Viral RNA was found in the majority of analyzed organs, and sublethally infected animals developed virus-specific neutralizing antibodies. Altogether, these results reveal IFNAR-KO mice as a new small animal model to study HeV and NiV pathogenesis, prophylaxis, and treatment and suggest the critical role of type I interferon signaling in the control of henipavirus infection.
Figures



References
-
- Murray K, Selleck P, Hooper P, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–7. - PubMed
-
- Chua KB, Bellini WJ, Rota PA, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–5. - PubMed
-
- Lo MK, Rota PA. The emergence of Nipah virus, a highly pathogenic paramyxovirus. J Clin Virol. 2008;43:396–400. - PubMed
-
- Tan CT, Goh KJ, Wong KT, et al. Relapsed and late-onset Nipah encephalitis. Ann Neurol. 2002;51:703–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

