ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans
- PMID: 23089736
- PMCID: PMC3519995
- DOI: 10.1038/mt.2012.223
ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans
Abstract
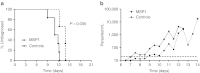
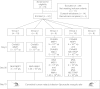
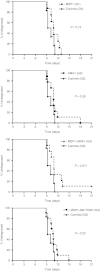
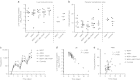
The induction of cellular immunity, in conjunction with antibodies, may be essential for vaccines to protect against blood-stage infection with the human malaria parasite Plasmodium falciparum. We have shown that prime-boost delivery of P. falciparum blood-stage antigens by chimpanzee adenovirus 63 (ChAd63) followed by the attenuated orthopoxvirus MVA is safe and immunogenic in healthy adults. Here, we report on vaccine efficacy against controlled human malaria infection delivered by mosquito bites. The blood-stage malaria vaccines were administered alone, or together (MSP1+AMA1), or with a pre-erythrocytic malaria vaccine candidate (MSP1+ME-TRAP). In this first human use of coadministered ChAd63-MVA regimes, we demonstrate immune interference whereby responses against merozoite surface protein 1 (MSP1) are dominant over apical membrane antigen 1 (AMA1) and ME-TRAP. We also show that induction of strong cellular immunity against MSP1 and AMA1 is safe, but does not impact on parasite growth rates in the blood. In a subset of vaccinated volunteers, a delay in time to diagnosis was observed and sterilizing protection was observed in one volunteer coimmunized with MSP1+AMA1-results consistent with vaccine-induced pre-erythrocytic, rather than blood-stage, immunity. These data call into question the utility of T cell-inducing blood-stage malaria vaccines and suggest that the focus should remain on high-titer antibody induction against susceptible antigen targets.
Figures




Comment in
-
Still seeking an effective "one-two" malaria vaccine punch.Mol Ther. 2012 Dec;20(12):2198-200. doi: 10.1038/mt.2012.241. Mol Ther. 2012. PMID: 23201615 Free PMC article. No abstract available.
References
-
- Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T., and, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. - PMC - PubMed
-
- Holder AA. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology. 2009;136:1445–1456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

