Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy
- PMID: 23090087
- PMCID: PMC3651893
- DOI: 10.1038/nrcardio.2012.145
Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy
Abstract
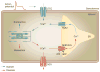
Cardiac myocyte function is dependent on the synchronized movements of Ca(2+) into and out of the cell, as well as between the cytosol and sarcoplasmic reticulum. These movements determine cardiac rhythm and regulate excitation-contraction coupling. Ca(2+) cycling is mediated by a number of critical Ca(2+)-handling proteins and transporters, such as L-type Ca(2+) channels (LTCCs) and sodium/calcium exchangers in the sarcolemma, and sarcoplasmic/endoplasmic reticulum calcium ATPase 2a (SERCA2a), ryanodine receptors, and cardiac phospholamban in the sarcoplasmic reticulum. The entry of Ca(2+) into the cytosol through LTCCs activates the release of Ca(2+) from the sarcoplasmic reticulum through ryanodine receptor channels and initiates myocyte contraction, whereas SERCA2a and cardiac phospholamban have a key role in sarcoplasmic reticulum Ca(2+) sequesteration and myocyte relaxation. Excitation-contraction coupling is regulated by phosphorylation of Ca(2+)-handling proteins. Abnormalities in sarcoplasmic reticulum Ca(2+) cycling are hallmarks of heart failure and contribute to the pathophysiology and progression of this disease. Correcting impaired intracellular Ca(2+) cycling is a promising new approach for the treatment of heart failure. Novel therapeutic strategies that enhance myocyte Ca(2+) homeostasis could prevent and reverse adverse cardiac remodeling and improve clinical outcomes in patients with heart failure.
Conflict of interest statement
R. J. Hajjar declares an association with the following company: Celladon Corporation. See the article online for full details of the relationship. The other authors declare no competing interests.
Figures

References
-
- de Giuli F, et al. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696,884 in the United Kingdom. Eur J Heart Fail. 2005;7:295–302. - PubMed
-
- Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112:3577–3583. - PubMed
-
- Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. 2009;95:1727–1731. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

