Optimum ribavirin exposure overcomes racial disparity in efficacy of peginterferon and ribavirin treatment for hepatitis C genotype 1
- PMID: 23090351
- PMCID: PMC3718255
- DOI: 10.1038/ajg.2012.306
Optimum ribavirin exposure overcomes racial disparity in efficacy of peginterferon and ribavirin treatment for hepatitis C genotype 1
Abstract
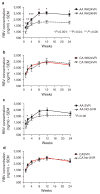
Objectives: Peginterferon and ribavirin treatment is less effective for hepatitis C virus (HCV) genotype 1 infections in African Americans (AA) compared with Caucasian Americans (CA). Host genetic variability near the interleukin-28B (IL28B) gene locus is partly responsible. We investigated the relationship between ribavirin drug exposure and week 24 and 72 (sustained virologic response, SVR) responses (undetected serum HCV RNA) in 71 AA and 74 CA with HCV genotype 1 who received >90% of the prescribed peginterferon and weight-based ribavirin (1,000 or 1,200 mg per day) from week 1 to 24.
Methods: Ribavirin plasma levels were measured at weeks 1, 2, 4, 8, 12 and 24; ribavirin area under the concentration vs. time curve (AUC) was calculated using the linear trapezoidal rule.
Results: Compared with CA, AA had lower week 24 (WK24VR) (57.8 vs. 78.1; P<0.05) and week 72 (SVR) (36.6% vs 54.8%; P<0.05) response rates. AA also had significantly lower ribavirin exposure (AUC) from week 1 to 12 (P<0.05). Ribavirin exposures ≥4,065 and ≥4,480 ng/ml/day in the first week (AUC(0-7)) were thresholds for WK24VR and SVR in receiver-operating characteristic curve analyses. AA were less likely to have a threshold ribavirin AUC(0-7) level than CA (P<0.05). There were no significant racial differences in WK24VR (AA: 77 vs. CA: 84%) and SVR (AA: 52 vs. CA: 60%) rates in patients who met the ribavirin AUC(0-7) thresholds. Ribavirin AUC(0-7) predicted WK24VR and SVR independently of IL28B single-nucleotide polymorphism rs12979860 genotype. Yet, achieving threshold AUC(0-7) levels increased response rates primarily in AA with the less favorable non-C/C genotypes.
Conclusions: Standard weight-based dosing leads to suboptimal ribavirin exposure in AA and contributes to the racial disparity in peginterferon and ribavirin treatment efficacy for HCV genotype 1.
Conflict of interest statement
The remaining authors declare no conflict of interest.
Figures




References
-
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C. N Engl J Med. 2002;347:975–82. - PubMed
-
- Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-hispanic whites. N Engl J Med. 2004;350:2265–71. - PubMed
-
- Conjeevaram HS, Fried MW, Jeffers LJ, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–7. - PubMed
-
- Hoofnagle JH, Wahed AS, Brown RS, Jr, et al. Early changes in Hepatitis C virus (HCV) levels in response to peginterferon and ribavirin treatment in patients with chronic HCV genotype 1 infection. J Infect Dis. 2009;199:1112–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK60329/DK/NIDDK NIH HHS/United States
- U01 DK60342/DK/NIDDK NIH HHS/United States
- U01 DK60349/DK/NIDDK NIH HHS/United States
- U01 DK060327/DK/NIDDK NIH HHS/United States
- U01 DK60324/DK/NIDDK NIH HHS/United States
- M01 RR016500/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- U01 DK60345/DK/NIDDK NIH HHS/United States
- R01 DK066920-01/DK/NIDDK NIH HHS/United States
- M01 RR000079/RR/NCRR NIH HHS/United States
- U01 DK060345/DK/NIDDK NIH HHS/United States
- M01 RR000645/RR/NCRR NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- 1 R21 DK078100-01/DK/NIDDK NIH HHS/United States
- U01 DK60344/DK/NIDDK NIH HHS/United States
- M01 RR16500/RR/NCRR NIH HHS/United States
- U01 DK60309/DK/NIDDK NIH HHS/United States
- K24 DK072036/DK/NIDDK NIH HHS/United States
- M01 RR00645/RR/NCRR NIH HHS/United States
- M01 RR00046/RR/NCRR NIH HHS/United States
- U01 DK060349/DK/NIDDK NIH HHS/United States
- U01 DK60327/DK/NIDDK NIH HHS/United States
- U01 DK60352/DK/NIDDK NIH HHS/United States
- U01 DK60341/DK/NIDDK NIH HHS/United States
- U01 DK060342/DK/NIDDK NIH HHS/United States
- M02 RR000079/RR/NCRR NIH HHS/United States
- U01 DK060341/DK/NIDDK NIH HHS/United States
- U01 DK060352/DK/NIDDK NIH HHS/United States
- U01 DK060335/DK/NIDDK NIH HHS/United States
- U01 DK60335/DK/NIDDK NIH HHS/United States
- U01 DK60346/DK/NIDDK NIH HHS/United States
- U01 DK060324/DK/NIDDK NIH HHS/United States
- U01 DK060309/DK/NIDDK NIH HHS/United States
- 1 K24 DK072036-01/DK/NIDDK NIH HHS/United States
- R21 DK078100/DK/NIDDK NIH HHS/United States
- U01 DK060344/DK/NIDDK NIH HHS/United States
- U01 DK060346/DK/NIDDK NIH HHS/United States
- U01 DK060340/DK/NIDDK NIH HHS/United States
- R01 DK066920/DK/NIDDK NIH HHS/United States
- U01 DK60340/DK/NIDDK NIH HHS/United States
- U01 DK060329/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

