High-resolution optical mapping of inflammatory macrophages following endovascular arterial injury
- PMID: 23090852
- PMCID: PMC3650124
- DOI: 10.1007/s11307-012-0599-2
High-resolution optical mapping of inflammatory macrophages following endovascular arterial injury
Abstract
Purpose: Inflammation following arterial injury mediates vascular restenosis, a leading cause of cardiovascular morbidity. Here we utilize intravital microscopy (IVM) and a dextran-coated nanosensor to spatially map inflammatory macrophages in vivo following endovascular injury of murine carotid arteries.
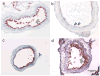
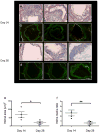
Procedures: C57Bl/6 mice (n = 23) underwent endovascular guidewire carotid arterial injury. At day 14 or day 28 post-injury, mice underwent fluorescence IVM, 24 h after injection with the near-infrared fluorescent macrophage nanosensor CLIO-VT680. Adventitial collagen was concomitantly imaged using second harmonic generation (SHG) IVM. Correlative fluorescence microscopy and immunohistochemistry were performed.
Results: Two-plane IVM reconstructions detected macrophage inflammation in the arterial wall that was elevated at day 14 compared to day 28 animals (P < 0.05). SHG-based collagen imaging of the outer arterial wall facilitated analysis of the macrophage-rich, inflamed neointima. Histological analyses and fluorescence microscopy data demonstrated increased macrophage infiltration in day 14 compared to day 28 neointima.
Conclusions: We demonstrate that the macrophage response to arterial injury can be imaged in vivo using IVM-based molecular imaging, and shows a higher macrophage influx at day 14 compared to day 28 post-injury.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures




Similar articles
-
In vivo nanoparticle assessment of pathological endothelium predicts the development of inflow stenosis in murine arteriovenous fistula.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):189-96. doi: 10.1161/ATVBAHA.114.304483. Epub 2014 Nov 13. Arterioscler Thromb Vasc Biol. 2015. PMID: 25395614 Free PMC article.
-
Adventitial fibroblast-derived vascular endothelial growth factor promotes vasa vasorum-associated neointima formation and macrophage recruitment.Cardiovasc Res. 2020 Mar 1;116(3):708-720. doi: 10.1093/cvr/cvz159. Cardiovasc Res. 2020. PMID: 31241138
-
Sam68 impedes the recovery of arterial injury by augmenting inflammatory response.J Mol Cell Cardiol. 2019 Dec;137:82-92. doi: 10.1016/j.yjmcc.2019.10.003. Epub 2019 Oct 19. J Mol Cell Cardiol. 2019. PMID: 31639388 Free PMC article.
-
Three-Dimensional Imaging Provides Detailed Atherosclerotic Plaque Morphology and Reveals Angiogenesis After Carotid Artery Ligation.Circ Res. 2020 Feb 28;126(5):619-632. doi: 10.1161/CIRCRESAHA.119.315804. Epub 2020 Jan 9. Circ Res. 2020. PMID: 31914850 Free PMC article.
-
Inflammation and vascular injury: basic discovery to drug development.Circ J. 2012;76(8):1811-8. doi: 10.1253/circj.cj-12-0801. Epub 2012 Jul 30. Circ J. 2012. PMID: 22785436 Free PMC article. Review.
Cited by
-
Imaging the pharmacology of nanomaterials by intravital microscopy: Toward understanding their biological behavior.Adv Drug Deliv Rev. 2017 Apr;113:61-86. doi: 10.1016/j.addr.2016.05.023. Epub 2016 Jun 4. Adv Drug Deliv Rev. 2017. PMID: 27266447 Free PMC article. Review.
-
In vivo detection of macrophage recruitment in hind-limb ischemia using a targeted near-infrared fluorophore.PLoS One. 2014 Jul 29;9(7):e103721. doi: 10.1371/journal.pone.0103721. eCollection 2014. PLoS One. 2014. PMID: 25072508 Free PMC article.
-
In vivo nanoparticle assessment of pathological endothelium predicts the development of inflow stenosis in murine arteriovenous fistula.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):189-96. doi: 10.1161/ATVBAHA.114.304483. Epub 2014 Nov 13. Arterioscler Thromb Vasc Biol. 2015. PMID: 25395614 Free PMC article.
-
ER Alpha Rapid Signaling Is Required for Estrogen Induced Proliferation and Migration of Vascular Endothelial Cells.PLoS One. 2016 Apr 1;11(4):e0152807. doi: 10.1371/journal.pone.0152807. eCollection 2016. PLoS One. 2016. PMID: 27035664 Free PMC article.
-
Mast cell activation and degranulation in acute artery injury: A target for post-operative therapy.FASEB J. 2023 Jul;37(7):e23029. doi: 10.1096/fj.202201745RR. FASEB J. 2023. PMID: 37310585 Free PMC article.
References
-
- Nakazawa G, Finn AV, Kolodgie FD, Virmani R. A review of current devices and a look at new technology: drug-eluting stents. Expert Rev Med Devices. 2009;6(1):33–42. - PubMed
-
- Edelman ER, Rogers C. Pathobiologic responses to stenting. Am J Cardiol. 1998;81:4E–6E. - PubMed
-
- Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–76. - PubMed
-
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;1340(2):115–26. - PubMed
-
- Serrano CV, Ramires JA, Venturinelli M, et al. Coronary angioplasty results in leucocyte and platelet activation with adhesion molecule expression. Evidence of inflammatory responses in coronary angioplasty. J Am Coll Cardiol. 1997;29:1276–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

