Simplified mechanism for new particle formation from methanesulfonic acid, amines, and water via experiments and ab initio calculations
- PMID: 23090988
- PMCID: PMC3503172
- DOI: 10.1073/pnas.1211878109
Simplified mechanism for new particle formation from methanesulfonic acid, amines, and water via experiments and ab initio calculations
Abstract
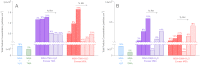
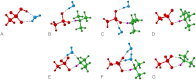
Airborne particles affect human health and significantly influence visibility and climate. A major fraction of these particles result from the reactions of gaseous precursors to generate low-volatility products such as sulfuric acid and high-molecular weight organics that nucleate to form new particles. Ammonia and, more recently, amines, both of which are ubiquitous in the environment, have also been recognized as important contributors. However, accurately predicting new particle formation in both laboratory systems and in air has been problematic. During the oxidation of organosulfur compounds, gas-phase methanesulfonic acid is formed simultaneously with sulfuric acid, and both are found in particles in coastal regions as well as inland. We show here that: (i) Amines form particles on reaction with methanesulfonic acid, (ii) water vapor is required, and (iii) particle formation can be quantitatively reproduced by a semiempirical kinetics model supported by insights from quantum chemical calculations of likely intermediate clusters. Such an approach may be more broadly applicable in models of outdoor, indoor, and industrial settings where particles are formed, and where accurate modeling is essential for predicting their impact on health, visibility, and climate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

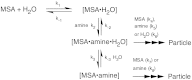

References
-
- Intergovernmental Panel on Climate Change. Climate change 2007: Synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. In: Pauchauri RK, Reisinger A, editors. The IPCC 4th Assessment Report. Geneva: IPCC; 2007. Core Writing Team.
-
- Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. - PubMed
-
- Finlayson-Pitts BJ, Pitts JN., Jr . Chemistry of the Upper and Lower Atmosphere—Theory Experiments and Applications. San Diego: Academic Press; 2000.
-
- Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. New York: Wiley Interscience; 2006.
-
- Phalen RF. Inhalation Studies: Foundations and Techniques. Boca Raton, FL: CRC; 1984.

