Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group
- PMID: 23091096
- PMCID: PMC3494838
- DOI: 10.1200/JCO.2011.41.5703
Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group
Erratum in
-
Errata.J Clin Oncol. 2015 Mar 1;33(7):814. doi: 10.1200/JCO.2015.61.1491. J Clin Oncol. 2015. PMID: 25721580 Free PMC article. No abstract available.
Abstract
Purpose: Chemotherapy with alternating vincristine-doxorubicin-cyclophosphamide and ifosfamide-etoposide cycles and primary tumor treatment with surgery and/or radiation therapy constitute the usual approach to localized Ewing sarcoma in North America. We tested whether chemotherapy intensification through interval compression could improve outcome.
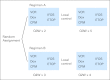
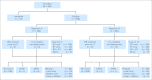
Patients and methods: This was a prospective, randomized controlled trial for patients younger than 50 years old with newly diagnosed localized extradural Ewing sarcoma. Patients assigned to standard and intensified treatment were to begin chemotherapy cycles every 21 and 14 days, respectively, provided an absolute neutrophil count greater than 750×10(6)/L and a platelet count greater than 75×10(9)/L. Patients received vincristine (2 mg/m2), doxorubicin (75 mg/m2), and cyclophosphamide (1.2 g/m2) alternating with ifosfamide (9 g/m2) and etoposide (500 mg/m2) for 14 cycles, with filgrastim (5 mg/kg per day; maximum, 300 mg) between cycles. Primary tumor treatment (surgery, radiation, or both) was to begin at week 13 (after four cycles in the standard arm and six cycles in the intensified arm). The primary end point was event-free survival (EFS). The study is registered at ClinicalTrials.gov (identifier: NCT00006734).
Results: Five hundred eighty-seven patients were enrolled and randomly assigned, and 568 patients were eligible, with 284 patients in each regimen. For all cycles, the median cycle interval for standard treatment was 21 days (mean, 22.45 days); for intensified treatment, the median interval was 15 days (mean, 17.29 days). EFS at a median of 5 years was 65% in the standard arm and 73% in the intensified arm (P=.048). The toxicity of the regimens was similar.
Conclusion: For localized Ewing sarcoma, chemotherapy administered every 2 weeks is more effective than chemotherapy administered every 3 weeks, with no increase in toxicity.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Chemotherapy. Intensified therapy for Ewing sarcoma.Nat Rev Clin Oncol. 2012 Dec;9(12):671. doi: 10.1038/nrclinonc.2012.200. Epub 2012 Nov 13. Nat Rev Clin Oncol. 2012. PMID: 23149897 No abstract available.
References
-
- Nesbit ME, Jr, Gehan EA, Burgert EO, Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: A long-term follow-up of the First Intergroup study. J Clin Oncol. 1990;8:1664–1674. - PubMed
-
- Smith MA, Ungerleider R, Horowitz ME, et al. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing's sarcoma. J Natl Cancer Inst. 1991;83:1460–1470. - PubMed
-
- Smith MA. The impact of doxorubicin dose intensity on survival of patients with Ewing's sarcoma. J Clin Oncol. 1991;9:889–891. - PubMed
-
- Burgert EO, Jr, Nesbit ME, Garnsey LA, et al. Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: Intergroup study IESS-II. J Clin Oncol. 1990;8:1514–1524. - PubMed
-
- Grier HE, Krailo M, Tarbell N, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

