Yield of screening for long-term complications using the children's oncology group long-term follow-up guidelines
- PMID: 23091100
- PMCID: PMC3515770
- DOI: 10.1200/JCO.2012.43.4951
Yield of screening for long-term complications using the children's oncology group long-term follow-up guidelines
Abstract
Purpose: The Children's Oncology Group Long-Term Follow-Up (COG-LTFU) Guidelines use consensus-based recommendations for exposure-driven, risk-based screening for early detection of long-term complications in childhood cancer survivors. However, the yield from these recommendations is not known.
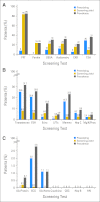
Methods: Survivors underwent COG-LTFU Guideline-directed screening. Yield was classified as negligible/negative (< 1%), intermediate (≥ 1% to < 10%), or high (≥ 10%). For long-term complications with high yield, logistic regression was used to identify subgroups more likely to screen positive.
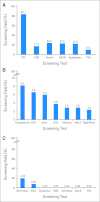
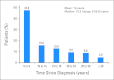
Results: Over the course of 1,188 clinic visits, 370 childhood cancer survivors (53% male; 47% Hispanic; 69% leukemia/lymphoma survivors; median age at diagnosis, 11.1 years [range, 0.3 to 21.9 years]; time from diagnosis, 10.5 years [range, 5 to 55.8 years]) underwent 4,992 screening tests. High-yield tests included thyroid function (hypothyroidism, 10.1%), audiometry (hearing loss, 22.6%), dual-energy x-ray absorptiometry scans (low bone mineral density [BMD], 23.2%), serum ferritin (iron overload, 24.0%), and pulmonary function testing/chest x-ray (pulmonary dysfunction, 84.1%). Regression analysis failed to identify subgroups more likely to result in high screening yield, with the exception of low BMD (2.5-fold increased risk for males [P = .04]; 3.3-fold increased risk for nonobese survivors [P = .01]). Screening tests with negligible/negative (< 1%) yield included complete blood counts (therapy-related leukemia), dipstick urinalysis for proteinuria and serum blood urea nitrogen/creatinine (glomerular defects), microscopic urinalysis for hematuria (hemorrhagic cystitis, bladder cancer), ECG (anthracycline-related conduction disorder), and hepatitis B and HIV serology.
Conclusion: Screening tests with a high yield are appropriate for risk groups targeted for screening by the COG-LTFU Guidelines. Elimination of screening tests with negligible/negative yield should be given consideration.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. - PubMed
-
- Armenian SH, Meadows AT, Bhatia S. Late effects of childhood cancer and its treatment. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. ed 6. Philadelphia, PA: Lippincott Raven; 2010. pp. 1431–1462.
-
- Landier W, Bhatia S. Cancer survivorship: A pediatric perspective. Oncologist. 2008;13:1181–1192. - PubMed
-
- Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

