ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation
- PMID: 23091165
- PMCID: PMC3501352
- DOI: 10.1084/jem.20111749
ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation
Abstract
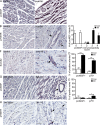
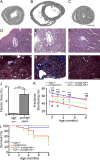
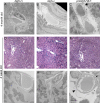
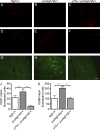
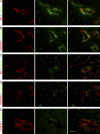
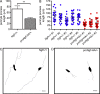
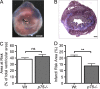
Treatment of acute cardiac ischemia focuses on reestablishment of blood flow in coronary arteries. However, impaired microvascular perfusion damages peri-infarct tissue, despite arterial patency. Identification of cytokines that induce microvascular dysfunction would provide new targets to limit microvascular damage. Pro-nerve growth factor (NGF), the precursor of NGF, is a well characterized cytokine in the brain induced by injury. ProNGF activates p75 neurotrophin receptor (p75(NTR)) and sortilin receptors to mediate proapoptotic responses. We describe induction of proNGF by cardiomyocytes, and p75(NTR) in human arterioles after fatal myocardial infarction, but not with unrelated pathologies. After mouse cardiac ischemia-reperfusion (I-R) injury, rapid up-regulation of proNGF by cardiomyocytes and p75(NTR) by microvascular pericytes is observed. To identify proNGF actions, we generated a mouse expressing a mutant Ngf allele with impaired processing of proNGF to mature NGF. The proNGF-expressing mouse exhibits cardiac microvascular endothelial activation, a decrease in pericyte process length, and increased vascular permeability, leading to lethal cardiomyopathy in adulthood. Deletion of p75(NTR) in proNGF-expressing mice rescues the phenotype, confirming the importance of p75(NTR)-expressing pericytes in the development of microvascular injury. Furthermore, deficiency in p75(NTR) limits infarct size after I-R. These studies identify novel, nonneuronal actions for proNGF and suggest that proNGF represents a new target to limit microvascular dysfunction.
Figures


References
-
- Bjarnegård M., Enge M., Norlin J., Gustafsdottir S., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fässler R., Betsholtz C. 2004. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 131:1847–1857 10.1242/dev.01080 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

