Controlling self-renewal and differentiation of stem cells via mechanical cues
- PMID: 23091358
- PMCID: PMC3471035
- DOI: 10.1155/2012/797410
Controlling self-renewal and differentiation of stem cells via mechanical cues
Abstract
The control of stem cell response in vitro, including self-renewal and lineage commitment, has been proved to be directed by mechanical cues, even in the absence of biochemical stimuli. Through integrin-mediated focal adhesions, cells are able to anchor onto the underlying substrate, sense the surrounding microenvironment, and react to its properties. Substrate-cell and cell-cell interactions activate specific mechanotransduction pathways that regulate stem cell fate. Mechanical factors, including substrate stiffness, surface nanotopography, microgeometry, and extracellular forces can all have significant influence on regulating stem cell activities. In this paper, we review all the most recent literature on the effect of purely mechanical cues on stem cell response, and we introduce the concept of "force isotropy" relevant to cytoskeletal forces and relevant to extracellular loads acting on cells, to provide an interpretation of how the effects of insoluble biophysical signals can be used to direct stem cells fate in vitro.
Figures


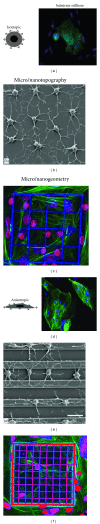
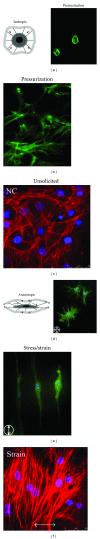
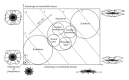
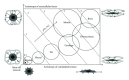
References
-
- Ding S, Schultz PG. A role for chemistry in stem cell biology. Nature Biotechnology. 2004;22(7):833–840. - PubMed
-
- Atala A, Lanza R, Thomson JA, Nerem RM. Principles of Regenerative Medicine. Vol. 1448. Burlington, Mass, USA: Elsevier; 2008.
-
- Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. The Lancet. 2008;372(9655):2023–2030. - PubMed
-
- Castillo AB, Jacobs CR. Mesenchymal stem cell mechanobiology. Current Osteoporosis Reports. 2010;8(2):98–104. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

