The inhibitory effect of Prunella vulgaris L. on aldose reductase and protein glycation
- PMID: 23091363
- PMCID: PMC3471066
- DOI: 10.1155/2012/928159
The inhibitory effect of Prunella vulgaris L. on aldose reductase and protein glycation
Abstract
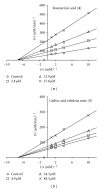
To evaluate the aldose reductase (AR) enzyme inhibitory ability of Prunella vulgaris L. extract, six compounds were isolated and tested for their effects. The components were subjected to in vitro bioassays to investigate their inhibitory assays using rat lens aldose reductase (rAR) and human recombinant AR (rhAR). Among them, caffeic acid ethylene ester showed the potent inhibition, with the IC(50) values of rAR and rhAR at 3.2 ± 0.55 μM and 12.58 ± 0.32 μM, respectively. In the kinetic analyses using Lineweaver-Burk plots of 1/velocity and 1/concentration of substrate, this compound showed noncompetitive inhibition against rhAR. Furthermore, it inhibited galactitol formation in a rat lens incubated with a high concentration of galactose. Also it has antioxidative as well as advanced glycation end products (AGEs) inhibitory effects. As a result, this compound could be offered as a leading compound for further study as a new natural products drug for diabetic complications.
Figures

References
-
- Moon HI, Jung JC, Lee J. Aldose reductase inhibitory effect by tectorigenin derivatives from Viola hondoensis. Bioorganic and Medicinal Chemistry. 2006;14(22):7592–7594. - PubMed
-
- Leal EC, Santiago AR, Ambrósio AF. Old and new drug targets in diabetic retinopathy: from biochemical changes to inflammation and neurodegeneration. Current Drug Targets: CNS and Neurological Disorders. 2005;4(4):421–434. - PubMed
-
- Kinoshita JH. Mechanisms initiating cataract formation. Investigative Ophthalmology. 1974;13(10):713–724. - PubMed
-
- Tomlinson DR, Stevens EJ, Diemel LT. Aldose reductase inhibitors and their potential for the treatment of diabetic complications. Trends in Pharmacological Sciences. 1994;15(8):293–297. - PubMed
-
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

