Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain
- PMID: 23091405
- PMCID: PMC3477677
- DOI: 10.7150/ijms.4706
Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain
Abstract
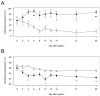
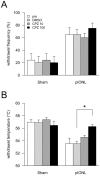
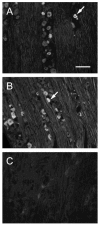
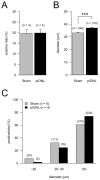
Trigeminal neuropathic pain is a facial pain syndrome associated with trigeminal nerve injury. However, the mechanism of trigeminal neuropathic pain is poorly understood. This study aimed to determine the role of transient receptor potential vanilloid 1 (TRPV1) in heat hyperalgesia in a trigeminal neuropathic pain model. We evaluated nociceptive responses to mechanical and heat stimuli using a partial infraorbital nerve ligation (pIONL) model. Withdrawal responses to mechanical and heat stimuli to vibrissal pads (VP) were assessed using von Frey filaments and a thermal stimulator equipped with a heat probe, respectively. Changes in withdrawal responses were measured after subcutaneous injection of the TRP channel antagonist capsazepine. In addition, the expression of TRPV1 in the trigeminal ganglia was examined. Mechanical allodynia and heat hyperalgesia were observed in VP by pIONL. Capsazepine suppressed heat hyperalgesia but not mechanical allodynia. The number of TRPV1-positive neurons in the trigeminal ganglia was significantly increased in the large-diameter-cell group. These results suggest that TRPV1 plays an important role in the heat hyperalgesia observed in the pIONL model.
Keywords: TRPV1; heat hyperalgesia.; trigeminal neuropathic pain.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain.J Pain. 2007 Jul;8(7):588-97. doi: 10.1016/j.jpain.2007.03.001. Epub 2007 May 3. J Pain. 2007. PMID: 17481957
-
Facial pain induces the alteration of transient receptor potential vanilloid receptor 1 expression in rat trigeminal ganglion.Neurosci Bull. 2007 Mar;23(2):92-100. doi: 10.1007/s12264-007-0013-2. Neurosci Bull. 2007. PMID: 17592531 Free PMC article.
-
Involvement of TRPV1 and TRPA1 in incisional intraoral and extraoral pain.J Dent Res. 2015 Mar;94(3):446-54. doi: 10.1177/0022034514565645. Epub 2015 Jan 9. J Dent Res. 2015. PMID: 25576470 Free PMC article.
-
Neural Pathways of Craniofacial Muscle Pain: Implications for Novel Treatments.J Dent Res. 2020 Aug;99(9):1004-1012. doi: 10.1177/0022034520919384. Epub 2020 May 6. J Dent Res. 2020. PMID: 32374638 Free PMC article. Review.
-
The Emerging Pro-Algesic Profile of Transient Receptor Potential Vanilloid Type 4.Rev Physiol Biochem Pharmacol. 2023;186:57-93. doi: 10.1007/112_2022_75. Rev Physiol Biochem Pharmacol. 2023. PMID: 36378366 Review.
Cited by
-
ThermoTRPs and Pain.Neuroscientist. 2016 Apr;22(2):171-87. doi: 10.1177/1073858414567884. Epub 2015 Jan 21. Neuroscientist. 2016. PMID: 25608689 Free PMC article. Review.
-
Targeting Pain-evoking Transient Receptor Potential Channels for the Treatment of Pain.Curr Neuropharmacol. 2013 Dec;11(6):652-63. doi: 10.2174/1570159X113119990040. Curr Neuropharmacol. 2013. PMID: 24396340 Free PMC article.
-
Heat Emitting Damage in Skin: A Thermal Pathway for Mechanical Algesia.Front Neurosci. 2021 Oct 28;15:780623. doi: 10.3389/fnins.2021.780623. eCollection 2021. Front Neurosci. 2021. PMID: 34776861 Free PMC article.
-
Peripheral afferents and spinal inhibitory system in dynamic and static mechanical allodynia.Pain. 2017 Dec;158(12):2285-2289. doi: 10.1097/j.pain.0000000000001055. Pain. 2017. PMID: 28885453 Free PMC article. Review. No abstract available.
-
Transient Receptor Potential (TRP) Ion Channels in Orofacial Pain.Mol Neurobiol. 2021 Jun;58(6):2836-2850. doi: 10.1007/s12035-021-02284-2. Epub 2021 Jan 29. Mol Neurobiol. 2021. PMID: 33515176 Free PMC article. Review.
References
-
- Türp JC, Gobetti JP. Trigeminal neuralgia versus atypical facial pain. A review of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:424–32. - PubMed
-
- Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123:64–74. - PubMed
-
- Eide PK, Rabben T. Trigeminal neuropathic pain: pathophysiological mechanisms examined by quantitative assessment of abnormal pain and sensory perception. Neurosurgery. 1998;43:1103–10. - PubMed
-
- Fried K, Bongenhielm U, Boissonade FM, Robinson PP. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–65. - PubMed
-
- Shinoda M, Kawashima K, Ozaki N, Asai H, Nagamine K, Sugiura Y. P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain. 2007;8:588–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

