Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell
- PMID: 23091420
- PMCID: PMC3473016
- DOI: 10.1371/journal.pbio.1001407
Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell
Abstract
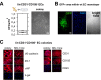
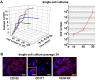
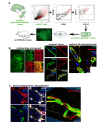
In adults, the growth of blood vessels, a process known as angiogenesis, is essential for organ growth and repair. In many disorders including cancer, angiogenesis becomes excessive. The cellular origin of new vascular endothelial cells (ECs) during blood vessel growth in angiogenic situations has remained unknown. Here, we provide evidence for adult vascular endothelial stem cells (VESCs) that reside in the blood vessel wall endothelium. VESCs constitute a small subpopulation within CD117+ (c-kit+) ECs capable of undergoing clonal expansion while other ECs have a very limited proliferative capacity. Isolated VESCs can produce tens of millions of endothelial daughter cells in vitro. A single transplanted c-kit-expressing VESC by the phenotype lin-CD31+CD105+Sca1+CD117+ can generate in vivo functional blood vessels that connect to host circulation. VESCs also have long-term self-renewal capacity, a defining functional property of adult stem cells. To provide functional verification on the role of c-kit in VESCs, we show that a genetic deficit in endothelial c-kit expression markedly decreases total colony-forming VESCs. In vivo, c-kit expression deficit resulted in impaired EC proliferation and angiogenesis and retardation of tumor growth. Isolated VESCs could be used in cell-based therapies for cardiovascular repair to restore tissue vascularization after ischemic events. VESCs also provide a novel cellular target to block pathological angiogenesis and cancer growth.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
On the hunt for vascular endothelial stem cells.PLoS Biol. 2012;10(10):e1001408. doi: 10.1371/journal.pbio.1001408. Epub 2012 Oct 16. PLoS Biol. 2012. PMID: 23091421 Free PMC article. No abstract available.
References
-
- Coultas L, Chawengsaksophak K, Rossant J (2005) Endothelial cells and VEGF in vascular development. Nature 438: 937–945. - PubMed
-
- Eichmann A, Yuan L, Moyon D, Lenoble F, Pardanaud L, et al. (2005) Vascular development: from precursor cells to branched arterial and venous networks. Int J Dev Biol 49: 259–267. - PubMed
-
- Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S (2003) VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood 101: 168–172. - PubMed
-
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. - PubMed
-
- Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438: 932–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

