Personalized medicine in breast cancer: a systematic review
- PMID: 23091538
- PMCID: PMC3468779
- DOI: 10.4048/jbc.2012.15.3.265
Personalized medicine in breast cancer: a systematic review
Abstract
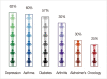
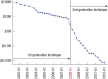
The recent advent of "-omics" technologies have heralded a new era of personalized medicine. Personalized medicine is referred to as the ability to segment heterogeneous subsets of patients whose response to a therapeutic intervention within each subset is homogeneous. This new paradigm in healthcare is beginning to affect both research and clinical practice. The key to success in personalized medicine is to uncover molecular biomarkers that drive individual variability in clinical outcomes or drug responses. In this review, we begin with an overview of personalized medicine in breast cancer and illustrate the most encountered statistical approaches in the recent literature tailored for uncovering gene signatures.
Keywords: Biomarker discovery; Breast neoplasms; Individualized medicine; Predictive biomarker; Prognostic biomarker.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7:201–204. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. - PubMed
-
- President's Council of Advisors on Science and Technolgy. Priorities for Personalized Medicine. Washington, DC: President's Council of Advisors on Science and Technolgy; 2008.
-
- Pfizer. Think Science Now Perspective. Approaches to Cancer Care: the Promise of Personalized Medicine. New York: Pfizer; 2010.
LinkOut - more resources
Full Text Sources

