Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote γ-irradiation-induced cell death in primary stem-like glioma cells
- PMID: 23091617
- PMCID: PMC3473017
- DOI: 10.1371/journal.pone.0047357
Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote γ-irradiation-induced cell death in primary stem-like glioma cells
Abstract
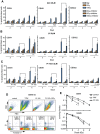
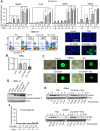
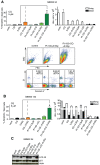
We asked whether inhibitors of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is highly active in cancer stem cells (CSCs) and upregulated in response to genotoxic treatments, promote γ-irradiationγIR)-induced cell death in highly radioresistant, patient-derived stem-like glioma cells (SLGCs). Surprisingly, in most cases the inhibitors did not promote γIR-induced cell death. In contrast, the strongly cytostatic Ly294002 and PI-103 even tended to reduce it. Since autophagy was induced we examined whether addition of the clinically applicable autophagy inhibitor chloroquine (CQ) would trigger cell death in SLGCs. Triple therapy with CQ at doses as low as 5 to 10 µM indeed caused strong apoptosis. At slightly higher doses, CQ alone strongly promoted γIR-induced apoptosis in all SLGC lines examined. The strong apoptosis in combinations with CQ was invariably associated with strong accumulation of the autophagosomal marker LC3-II, indicating inhibition of late autophagy. Thus, autophagy-promoting effects of PI3K/Akt pathway inhibitors apparently hinder cell death induction in γ-irradiated SLGCs. However, as we show here for the first time, the late autophagy inhibitor CQ strongly promotes γIR-induced cell death in highly radioresistant CSCs, and triple combinations of CQ, γIR and a PI3K/Akt pathway inhibitor permit reduction of the CQ dose required to trigger cell death.
Conflict of interest statement
Figures




References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996. - PubMed
-
- Maugeri-Sacca M, Vigneri P, De Maria R (2011) Cancer Stem Cells and Chemosensitivity. Clin Cancer Res 17: 4942–4947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

