Drug-induced liver injury: present and future
- PMID: 23091804
- PMCID: PMC3467427
- DOI: 10.3350/cmh.2012.18.3.249
Drug-induced liver injury: present and future
Abstract
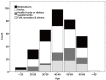
Liver injury due to prescription and nonprescription medications is a growing medical, scientific, and public health problem. Worldwide, the estimated annual incidence rate of drug-induced liver injury (DILI) is 13.9-24.0 per 100,000 inhabitants. DILI is one of the leading causes of acute liver failure in the US. In Korea, the annual extrapolated incidence of cases hospitalized at university hospital is 12/100,000 persons/year. Most cases of DILI are the result of idiosyncratic metabolic responses or unexpected reactions to medication. There is marked geographic variation in relevant agents; antibiotics, anticonvulsants, and psychotropic drugs are the most common offending agents in the West, whereas in Asia, 'herbs' and 'health foods or dietary supplements' are more common. Different medical circumstances also cause discrepancy in definition and classification of DILI between West and Asia. In the concern of causality assessment, the application of the Roussel Uclaf Causality Assessment Method (RUCAM) scale frequently undercounts the cases caused by 'herbs' due to a lack of previous information and incompatible time criteria. Therefore, a more objective and reproducible tool that could be used for the diagnosis of DILI caused by 'herbs' is needed in Asia. In addition, a reporting system similar to the Drug-Induced Liver Injury Network (DILIN) in the US should be established as soon as possible in Asia.
Keywords: Asia; Drug-Induced Liver Injury; Herbal Medicine.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures
References
-
- Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007;102:558–562. - PubMed
-
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–2404. - PubMed
-
- Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008;24:287–297. - PubMed
-
- Au JS, Navarro VJ, Rossi S. Review article: Drug-induced liver injury-its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther. 2011;34:11–20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical