Evidence of delayed gastrointestinal syndrome in high-dose irradiated mice
- PMID: 23091877
- PMCID: PMC3551349
- DOI: 10.1097/hp.0b013e31826530e2
Evidence of delayed gastrointestinal syndrome in high-dose irradiated mice
Abstract
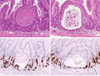
The acute effects of irradiation on the gastrointestinal (GI) system are well documented, but the longer-term effects are less well known. Increased incidence of adenocarcinoma has been noted, but apart from descriptions of fibrosis, the development of other pathologies specific to survivors of acute radiation is poorly understood. Samples were taken from C57BL/6 mice irradiated with partial-body irradiation where the thorax, head, and forelimbs were shielded (i.e., sparing 40% of the bone marrow). Tissue from age-matched controls was also collected. There were clear pathological changes in the intestine associated with DEARE (Delayed Effects of Acute Radiation Exposure) at doses greater than 12 Gy, with a dose-related increase in observed pathologies. Mice maintained on the synthetic antibiotic ciprofloxacin during the acute phase (days 4 to 20), however, had a lower or delayed incidence of symptoms. After 20 d, mice developed structures similar to early adenomas. Abnormally high levels of apoptotic and mitotic cells were present in some crypts, along with the early adenomas, suggesting tissue regeneration and areas of deregulated cell turnover. Over time, there was inhibited crypt cell proliferation in animals with advanced symptoms, a blunting of the crypts and villi, and an enlargement of villus girth, with an increasingly acellular and fibrotic extracellular matrix (a characteristic that has been demonstrated previously in aging mice). Together these changes may lead to a reduced functional surface area and less motile intestine. These observations are similar to those seen in geriatric animals, suggesting a premature aging of the GI tract.
Figures







References
-
- Boggs DR. The total marrow mass of the mouse: a simplified method of measurement. Am J Hematol. 1984;16(3):277–286. - PubMed
-
- Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Intl J Radiat Oncol Biol Phys. 1995;31(5):1213–1236. - PubMed
-
- Dubios A, Walker RI. Prospects for management of gastrointestinal injury associated with the acute radiation syndrome. Gastroenterology. 1988;95:500–507. - PubMed
-
- Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res. 2005;163(5):479–487. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

