Host responses in tissue repair and fibrosis
- PMID: 23092186
- PMCID: PMC3789589
- DOI: 10.1146/annurev-pathol-020712-163930
Host responses in tissue repair and fibrosis
Abstract
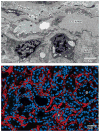
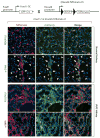
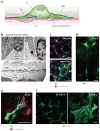
Myofibroblasts accumulate in the spaces between organ structures and produce extracellular matrix (ECM) proteins, including collagen I. They are the primary "effector" cells in tissue remodeling and fibrosis. Previously, leukocyte progenitors termed fibrocytes and myofibroblasts generated from epithelial cells through epithelial-to-mesenchymal transition (EMT) were considered the primary sources of ECM-producing myofibroblasts in injured tissues. However, genetic fate mapping experiments suggest that mesenchyme-derived cells, known as resident fibroblasts, and pericytes are the primary precursors of scar-forming myofibroblasts, whereas epithelial cells, endothelial cells, and myeloid leukocytes contribute to fibrogenesis predominantly by producing key fibrogenic cytokines and by promoting cell-to-cell communication. Numerous cytokines derived from T cells, macrophages, and other myeloid cell populations are important drivers of myofibroblast differentiation. Monocyte-derived cell populations are key regulators of the fibrotic process: They act as a brake on the processes driving fibrogenesis, and they dismantle and degrade established fibrosis. We discuss the origins, modes of activation, and fate of myofibroblasts in various important fibrotic diseases and describe how manipulation of macrophage activation could help ameliorate fibrosis.
Figures



References
-
- Burmolle M, Thomsen TR, Fazli M, Dige I, Christensen L, et al. Biofilms in chronic infections—a matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010;59:324–36. - PubMed
Publication types
MeSH terms
Grants and funding
- HL067967/HL/NHLBI NIH HHS/United States
- R01 DK093493/DK/NIDDK NIH HHS/United States
- R01 HL067967/HL/NHLBI NIH HHS/United States
- ZIA AI000829/ImNIH/Intramural NIH HHS/United States
- DK84077/DK/NIDDK NIH HHS/United States
- R01 DK084077/DK/NIDDK NIH HHS/United States
- HL107181/HL/NHLBI NIH HHS/United States
- P50 HL107181/HL/NHLBI NIH HHS/United States
- DK87389/DK/NIDDK NIH HHS/United States
- ZIA AI001019/ImNIH/Intramural NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- RC1 DK087389/DK/NIDDK NIH HHS/United States
- DK93493/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

