From the chemistry of epoxy-sugar nucleosides to the discovery of anti-HIV agent 4'-ethynylstavudine-Festinavir
- PMID: 23092278
- PMCID: PMC3711117
- DOI: 10.2174/1381612811319100011
From the chemistry of epoxy-sugar nucleosides to the discovery of anti-HIV agent 4'-ethynylstavudine-Festinavir
Abstract
Branched sugar nucleosides have attracted much attention due to their biological activities. We have demonstrated that epoxysugar nucleosides serve as versatile precursor for the stereo-defined synthesis of these nucleoside derivatives on the basis of its ring opening with organoaluminum or organosilicon reagents. In this review article, novel methods for the synthesis of nucleoside analogues branched at the 1' and 4'-position will be described. During this study, we could discover an anti-HIV agent, 4'-ethynylstavudine (Festinavir). Festinavir showed more potent anti-HIV activity than the parent compound stavudine (d4T). Other significant properties of Festinavir are as follows: 1) much less toxic to various cells and also to mitochondorial DNA synthesis than d4T, 2) better substrate for human thymidine kinase than d4T, 3) resistant not only to chemical glycosidic bond cleavage but also to catabolism by thymidine phosphorylase, 4) the activity improves in the presence of a major mutation, K103N, associated with resistance to non-nucleoside reverse transcriptase inhibitors. Detailed profile of the antiviral activities, biology and pharmacology of Festinavir are also described.
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Figures

















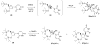













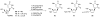
References
-
- [Accessed on 02/11/2012];Global report: UNAIDS report on the global AIDS endemic. 2010 http://www.unaids.org/globalreport/global_report.htm.
-
- Matsuda A. Recent development of anti-HIV nucleosides. J Synth Org Chem Jpn. 1990;48(10):907–20.
-
- Ichikawa E, Kato K. Sugar-modified nucleosides in past 10 years, a review. Current Medicinal Chemistry. 2001;8(4):385–423. - PubMed
-
- Huryn DM, Okabe M. AIDS-driven nucleoside chemistry. Chem Rev. 1992;92(8):1745–68.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

