Rationale, design, methodology and sample characteristics for the Vietnam pre-conceptual micronutrient supplementation trial (PRECONCEPT): a randomized controlled study
- PMID: 23092451
- PMCID: PMC3533960
- DOI: 10.1186/1471-2458-12-898
Rationale, design, methodology and sample characteristics for the Vietnam pre-conceptual micronutrient supplementation trial (PRECONCEPT): a randomized controlled study
Abstract
Background: Low birth weight and maternal anemia remain intractable problems in many developing countries. The adequacy of the current strategy of providing iron-folic acid (IFA) supplements only during pregnancy has been questioned given many women enter pregnancy with poor iron stores, the substantial micronutrient demand by maternal and fetal tissues, and programmatic issues related to timing and coverage of prenatal care. Weekly IFA supplementation for women of reproductive age (WRA) improves iron status and reduces the burden of anemia in the short term, but few studies have evaluated subsequent pregnancy and birth outcomes.The Preconcept trial aims to determine whether pre-pregnancy weekly IFA or multiple micronutrient (MM) supplementation will improve birth outcomes and maternal and infant iron status compared to the current practice of prenatal IFA supplementation only. This paper provides an overview of study design, methodology and sample characteristics from baseline survey data and key lessons learned.
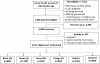
Methods/design: We have recruited 5011 WRA in a double-blind stratified randomized controlled trial in rural Vietnam and randomly assigned them to receive weekly supplements containing either: 1) 2800 μg folic acid 2) 60 mg iron and 2800 μg folic acid or 3) MM. Women who become pregnant receive daily IFA, and are being followed through pregnancy, delivery, and up to three months post-partum. Study outcomes include birth outcomes and maternal and infant iron status. Data are being collected on household characteristics, maternal diet and mental health, anthropometry, infant feeding practices, morbidity and compliance.
Discussion: The study is timely and responds to the WHO Global Expert Consultation which identified the need to evaluate the long term benefits of weekly IFA and MM supplementation in WRA. Findings will generate new information to help guide policy and programs designed to reduce the burden of anemia in women and children and improve maternal and child health outcomes in resource poor settings.
Trial registration: NCT01665378.
Figures
References
-
- UNICEF/WHO. Low Birthweight. New York, NY: Country, regional and global estimates; 2004.
-
- UNICEF. State of the Worlds Children 209. New York, NY: Maternal and Newborn Health; 2009. 2009.
-
- De Benoist B, McLean E, Egli I, Cogswell M. Worldwide Prevalence of Anaemia 1993-2005: WHO Global Database on Anemia. Geneva: WHO; 2008.
-
- Imdad A, Bhutta ZA. Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatr Perinat Epidemiol. 2012;26(Supplement 1):168–177. - PubMed
-
- Pena-Rosas JP, Viteri F. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database Syst Rev. 2009;19(3):CD004736. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical