Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy
- PMID: 23092791
- PMCID: PMC3636692
- DOI: 10.1369/0022155412467635
Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy
Abstract
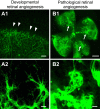
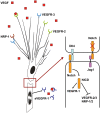
Endothelial tip cells are leading cells at the tips of vascular sprouts coordinating multiple processes during angiogenesis. In the developing retina, tip cells play a tightly controlled, timely role in angiogenesis. In contrast, excessive numbers of tip cells are a characteristic of the chaotic pathological blood vessels in proliferative retinopathies. Tip cells control adjacent endothelial cells in a hierarchical manner to form the stalk of the sprouting vessel, using, among others, the VEGF-DLL-Notch signaling pathway, and recruit pericytes. Tip cells are guided toward avascular areas by signals from the local extracellular matrix that are released by cells from the neuroretina such as astrocytes. Recently, tip cells were identified in endothelial cell cultures, enabling identification of novel molecular markers and mechanisms involved in tip cell biology. These mechanisms are relevant for understanding proliferative retinopathies. Agents that primarily target tip cells can block pathological angiogenesis in the retina efficiently and safely without adverse effects. A striking example is platelet-derived growth factor, which was recently shown to be an efficacious additional target in the treatment of retinal neovascularization. Here we discuss these and other tip cell-based strategies with respect to their potential to treat patients with ocular diseases dominated by neovascularization.
Conflict of interest statement
Figures

References
-
- Bai YY, Bai X, Wang ZY, Zhang XF, Ruan CG, Miao JC. 2011. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp Mol Pathol. 91:471–477 - PubMed
-
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. 2009. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135 - PubMed
-
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. 2012. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 484:110–114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

