Analysis of drug interactions with modified proteins by high-performance affinity chromatography: binding of glibenclamide to normal and glycated human serum albumin
- PMID: 23092871
- PMCID: PMC3489001
- DOI: 10.1016/j.chroma.2012.09.091
Analysis of drug interactions with modified proteins by high-performance affinity chromatography: binding of glibenclamide to normal and glycated human serum albumin
Abstract
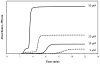
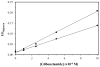
High-performance affinity chromatography (HPAC) was used to examine the changes in binding that occur for the sulfonylurea drug glibenclamide with human serum albumin (HSA) at various stages of glycation for HSA. Frontal analysis on columns containing normal HSA or glycated HSA indicated glibenclamide was interacting through both high affinity sites (association equilibrium constant, K(a), 1.4-1.9 × 10(6)M(-1) at pH 7.4 and 37 °C) and lower affinity sites (K(a), 4.4-7.2 × 10(4)M(-1)). Competition studies were used to examine the effect of glycation at specific binding sites of HSA. An increase in affinity of 1.7- to 1.9-fold was seen at Sudlow site I with moderate to high levels of glycation. An even larger increase of 4.3- to 6.0-fold in affinity was noted at Sudlow site II for all of the tested samples of glycated HSA. A slight decrease in affinity may have occurred at the digitoxin site, but this change was not significant for any individual glycated HSA sample. These results illustrate how HPAC can be used as tool for examining the interactions of relatively non-polar drugs like glibenclamide with modified proteins and should lead to a more complete understanding of how glycation can alter the binding of drugs in blood.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Analysis of glipizide binding to normal and glycated human serum albumin by high-performance affinity chromatography.Anal Bioanal Chem. 2015 Jul;407(18):5309-21. doi: 10.1007/s00216-015-8688-0. Epub 2015 Apr 26. Anal Bioanal Chem. 2015. PMID: 25912461 Free PMC article.
-
Review: Glycation of human serum albumin.Clin Chim Acta. 2013 Oct 21;425:64-76. doi: 10.1016/j.cca.2013.07.013. Epub 2013 Jul 24. Clin Chim Acta. 2013. PMID: 23891854 Free PMC article. Review.
-
Analysis of multi-site drug-protein interactions by high-performance affinity chromatography: Binding by glimepiride to normal or glycated human serum albumin.J Chromatogr A. 2015 Aug 21;1408:133-44. doi: 10.1016/j.chroma.2015.07.012. Epub 2015 Jul 6. J Chromatogr A. 2015. PMID: 26189669 Free PMC article.
-
High-performance affinity chromatography and the analysis of drug interactions with modified proteins: binding of gliclazide with glycated human serum albumin.Anal Bioanal Chem. 2011 Nov;401(9):2811-9. doi: 10.1007/s00216-011-5382-8. Epub 2011 Sep 16. Anal Bioanal Chem. 2011. PMID: 21922305 Free PMC article.
-
Characterization of drug interactions with serum proteins by using high-performance affinity chromatography.Curr Drug Metab. 2011 May;12(4):313-28. doi: 10.2174/138920011795202938. Curr Drug Metab. 2011. PMID: 21395530 Free PMC article. Review.
Cited by
-
Rapid screening of drug-protein binding using high-performance affinity chromatography with columns containing immobilized human serum albumin.J Anal Methods Chem. 2013;2013:439039. doi: 10.1155/2013/439039. Epub 2013 Mar 28. J Anal Methods Chem. 2013. PMID: 23607050 Free PMC article.
-
Analysis of glipizide binding to normal and glycated human serum albumin by high-performance affinity chromatography.Anal Bioanal Chem. 2015 Jul;407(18):5309-21. doi: 10.1007/s00216-015-8688-0. Epub 2015 Apr 26. Anal Bioanal Chem. 2015. PMID: 25912461 Free PMC article.
-
Review: Glycation of human serum albumin.Clin Chim Acta. 2013 Oct 21;425:64-76. doi: 10.1016/j.cca.2013.07.013. Epub 2013 Jul 24. Clin Chim Acta. 2013. PMID: 23891854 Free PMC article. Review.
-
Chromatographic studies of chlorpropamide interactions with normal and glycated human serum albumin based on affinity microcolumns.J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Oct 15;1097-1098:64-73. doi: 10.1016/j.jchromb.2018.09.001. Epub 2018 Sep 4. J Chromatogr B Analyt Technol Biomed Life Sci. 2018. PMID: 30205233 Free PMC article.
-
[Advances in chromatography in the study of drug-plasma protein interactions].Se Pu. 2021 Oct;39(10):1077-1085. doi: 10.3724/SP.J.1123.2021.06028. Se Pu. 2021. PMID: 34505429 Free PMC article. Review. Chinese.
References
-
- International Diabetes Federation. IDF Diabetes Atlas. 5. Chap 2 Brussels, Belgium: International Diabetes Federation; 2011.
-
- National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2011. US Centers for Disease Control and Prevention; Atlanta, GA: 2011. pp. 1–12.
-
- Nelson DL, Cox M. Lehninger Principles of Biochemistry. Chap 23 Freeman; New York: 2005.
-
- Skillman TG, Feldman JM. Am J Med. 1981;70:36. - PubMed
-
- Amidon GL, Lennernas H, Shah VP, Crison JR. Pharm Res. 1995;12:413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

