Diffuse cerebral language representation in tuberous sclerosis complex
- PMID: 23092910
- PMCID: PMC3574215
- DOI: 10.1016/j.eplepsyres.2012.09.011
Diffuse cerebral language representation in tuberous sclerosis complex
Abstract
Introduction: Tuberous sclerosis complex (TSC) is a multisystem genetic disorder affecting multiple organs, including the brain, and very often associated with epileptic activity. Language acquisition and development seems to be altered in a significant proportion of patients with TSC. In the present study, we used magnetoencephalography (MEG) to investigate spatiotemporal cerebral language processing in subjects with TSC and epilepsy during a reading semantic decision task, compared to healthy control participants.
Methods: Fifteen patients with TSC and 31 healthy subjects performed a lexico-semantic decision task during MEG recording. Minimum-norm estimates (MNE) were computed allowing identification of cerebral generators of language evoked fields (EF) in each subject.
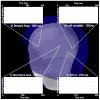
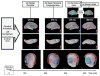
Results: Source analysis of the language EF demonstrated early bilateral medial occipital activation (125ms) followed by a fusiform gyrus activation around 135ms. At 270ms post stimuli presentation, a strong cerebral activation was recorded in the left basal temporal language area. Finally, cerebral activations were measured in Wernicke's area followed by Broca's area. The healthy control group showed larger and earlier language activations in Broca and Wernicke's areas compared to TSC patients. Moreover, cerebral activation from Broca's area was greater than activation from Wernicke's area in both groups, but this difference between anterior and posterior regions was smaller in the TSC group. Finally, the activation latency difference between Broca and Wernicke's areas was greater in healthy controls than in TSC patients, which shows that activations in these areas are more serial in control subjects compared to TSC patients in whom activations occur more simultaneously.
Conclusions: This is the first study to investigate cerebral language pattern in patients with TSC. Compared to healthy controls, atypical neuromagnetic language responses may reflect cerebral reorganization in these patients in response to early epileptogenic activity or presence at birth of multiple brain lesions.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Temporal and topographical characterization of language cortices using intraoperative optical intrinsic signals.Neuroimage. 2000 Jul;12(1):41-54. doi: 10.1006/nimg.2000.0597. Neuroimage. 2000. PMID: 10875901
-
Decreased language laterality in tuberous sclerosis complex: a relationship between language dominance and tuber location as well as history of epilepsy.Epilepsy Behav. 2012 Sep;25(1):36-41. doi: 10.1016/j.yebeh.2012.06.013. Epub 2012 Aug 17. Epilepsy Behav. 2012. PMID: 22980079 Free PMC article.
-
High-resolution MEG source imaging approach to accurately localize Broca's area in patients with brain tumor or epilepsy.Clin Neurophysiol. 2016 May;127(5):2308-16. doi: 10.1016/j.clinph.2016.02.007. Epub 2016 Feb 17. Clin Neurophysiol. 2016. PMID: 27072104
-
[Epilepsy surgery in children with tuberous sclerosis complex].No To Hattatsu. 2014 Jul;46(4):257-63. No To Hattatsu. 2014. PMID: 25154221 Review. Japanese.
-
Language Mapping With Magnetoencephalography: An Update on the Current State of Clinical Research and Practice With Considerations for Clinical Practice Guidelines.J Clin Neurophysiol. 2020 Nov;37(6):554-563. doi: 10.1097/WNP.0000000000000489. J Clin Neurophysiol. 2020. PMID: 33165228 Review.
Cited by
-
Brain language networks and cognitive outcomes in children with frontotemporal lobe epilepsy.Front Hum Neurosci. 2023 Oct 27;17:1253529. doi: 10.3389/fnhum.2023.1253529. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37964801 Free PMC article.
-
Altered cortical excitability in tuberous sclerosis and the effect of mTOR inhibitors: An intracranial electrical stimulation study.Clin Neurophysiol. 2025 Apr;172:1-9. doi: 10.1016/j.clinph.2025.02.001. Epub 2025 Feb 4. Clin Neurophysiol. 2025. PMID: 39947023
-
Understanding speech and language in tuberous sclerosis complex.Front Hum Neurosci. 2023 Jun 1;17:1149071. doi: 10.3389/fnhum.2023.1149071. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37323931 Free PMC article. Review.
-
Resting-State fMRI Networks in Children with Tuberous Sclerosis Complex.J Neuroimaging. 2019 Nov;29(6):750-759. doi: 10.1111/jon.12653. Epub 2019 Jul 14. J Neuroimaging. 2019. PMID: 31304656 Free PMC article.
-
Resective Surgery for Double Epileptic Foci Overlapping Anterior and Posterior Language Areas: A Case of Epilepsy With Tuberous Sclerosis Complex.Front Neurol. 2018 May 17;9:343. doi: 10.3389/fneur.2018.00343. eCollection 2018. Front Neurol. 2018. PMID: 29867747 Free PMC article.
References
-
- Bowyer SM, Moran JE, Weiland BJ, Mason KM, Greenwald ML, Smith BJ, Barkley GL, Tepley N. Language laterality determined by MEG mapping with MR-FOCUSS. Epilepsy Behav. 2005;6:235–241. - PubMed
-
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. - PubMed
-
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

