The dynamics of the mitochondrial organelle as a potential therapeutic target
- PMID: 23093069
- PMCID: PMC3597376
- DOI: 10.1038/jcbfm.2012.158
The dynamics of the mitochondrial organelle as a potential therapeutic target
Abstract
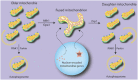
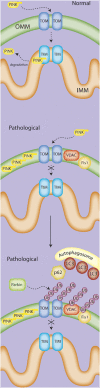
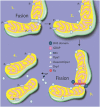
Mitochondria play a central role in cell fate after stressors such as ischemic brain injury. The convergence of intracellular signaling pathways on mitochondria and their release of critical factors are now recognized as a default conduit to cell death or survival. Besides the individual processes that converge on or emanate from mitochondria, a mitochondrial organellar response to changes in the cellular environment has recently been described. Whereas mitochondria have previously been perceived as a major center for cellular signaling, one can postulate that the organelle's dynamics themselves affect cell survival. This brief perspective review puts forward the concept that disruptions in mitochondrial dynamics--biogenesis, clearance, and fission/fusion events--may underlie neural diseases and thus could be targeted as neuroprotective strategies in the context of ischemic injury. To do so, we present a general overview of the current understanding of mitochondrial dynamics and regulation. We then review emerging studies that correlate mitochondrial biogenesis, mitophagy, and fission/fusion events with neurologic disease and recovery. An overview of the system as it is currently understood is presented, and current assessment strategies and their limitations are discussed.
Figures

References
-
- Goldenthal MJ, Marin-Garcia J. Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem. 2004;262:1–16. - PubMed
-
- Akepati VR, Muller EC, Otto A, Strauss HM, Portwich M, Alexander C. Characterization of OPA1 isoforms isolated from mouse tissues. J Neurochem. 2008;106:372–383. - PubMed
-
- Dennis EA, Kennedy EP. Intracellular sites of lipid synthesis and the biogenesis of mitochondria. J Lipid Res. 1972;13:263–267. - PubMed
-
- Michel S, Wanet A, De Pauw A, Rommelaere G, Arnould T, Renard P. Crosstalk between mitochondrial (dys)function and mitochondrial abundance. J Cell Physiol. 2012;227:2297–2310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

