Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma
- PMID: 23093355
- PMCID: PMC3594060
- DOI: 10.1007/s00270-012-0481-2
Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma
Abstract
Purpose: Intermediate-stage hepatocellular carcinoma (HCC) is usually treated with locoregional therapy using transarterial chemoembolization (TACE). Transarterial radioembolization (TARE) using β-emitting yttrium-90 integral to the glass matrix of the microspheres is an alternative to TACE. This retrospective case-control study compared the outcomes and safety of TARE versus TACE in patients with unresectable HCC.
Materials and methods: Patients with unresectable HCC without portal vein thrombosis treated with TARE between 2005 and 2008 (n = 61) were retrospectively frequency-matched by age, sex, and liver dysfunction with TACE-treated patients (n = 55) in the Mayo Clinic Hepatobiliary Neoplasia Registry. Imaging studies were reviewed, and clinical and safety outcomes were abstracted from the medical records.
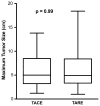
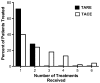
Results: Complete tumor response was more common after TARE (12 %) than after TACE (4 %) (p = 0.17). When complete response was combined with partial response and stable disease, there was no difference between TARE and TACE. Median survival did not differ between the two groups (15.0 months for TARE and 14.4 months for TACE; p = 0.47). Two-year survival rates were 30 % for TARE and 24 % for TACE. TARE patients received fewer treatments (p < 0.001). Fifty-nine (97 %) TARE patients received outpatient treatment. In contrast, 53 (98 %) TACE patients were hospitalized for ≥1 day (p < 0.001). Compared with TACE, TARE was more likely to induce fatigue (p = 0.003) but less likely to cause fever (p = 0.02).
Conclusion: There was no significant difference in efficacy between TARE and TACE. TARE patients reported more fatigue but had less fever than TACE patients. Treatment with TARE required less hospitalization than treatment with TACE. These findings require confirmation in randomized trials.
Conflict of interest statement
Figures
References
-
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–754. - PubMed
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. - PubMed
-
- Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4(7):424–432. - PubMed
-
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona 2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous