Exploring Ty1 retrotransposon RNA structure within virus-like particles
- PMID: 23093595
- PMCID: PMC3592414
- DOI: 10.1093/nar/gks983
Exploring Ty1 retrotransposon RNA structure within virus-like particles
Abstract
Ty1, a long terminal repeat retrotransposon of Saccharomyces, is structurally and functionally related to retroviruses. However, a differentiating aspect between these retroelements is the diversity of the replication strategies used by long terminal repeat retrotransposons. To understand the structural organization of cis-acting elements present on Ty1 genomic RNA from the GAG region that control reverse transcription, we applied chemoenzymatic probing to RNA/tRNA complexes assembled in vitro and to the RNA in virus-like particles. By comparing different RNA states, our analyses provide a comprehensive structure of the primer-binding site, a novel pseudoknot adjacent to the primer-binding sites, three regions containing palindromic sequences that may be involved in RNA dimerization or packaging and candidate protein interaction sites. In addition, we determined the impact of a novel form of transposon control based on Ty1 antisense transcripts that associate with virus-like particles. Our results support the idea that antisense RNAs inhibit retrotransposition by targeting Ty1 protein function rather than annealing with the RNA genome.
Figures
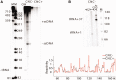
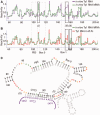

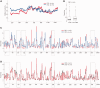
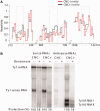
References
-
- Voytas DF, Boeke JD. Ty1 and Ty5 of Saccharomyces cerevisiae. In: Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washington, DC: ASM Press; 2002. pp. 614–630.
-
- Lesage P, Todeschini A. Happy together: the life and times of Ty retrotransposons and their hosts. Cytogenet. Genome Res. 2005;110:70–90. - PubMed

