Intrinsic reaction-cycle time scale of Na+,K+-ATPase manifests itself in the lipid-protein interactions of nonequilibrium membranes
- PMID: 23093677
- PMCID: PMC3494963
- DOI: 10.1073/pnas.1209909109
Intrinsic reaction-cycle time scale of Na+,K+-ATPase manifests itself in the lipid-protein interactions of nonequilibrium membranes
Abstract
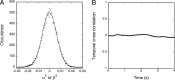
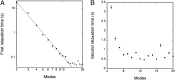
Interaction between integral membrane proteins and the lipid-bilayer component of biological membranes is expected to mutually influence the proteins and the membrane. We present quantitative evidence of a manifestation of the lipid-protein interactions in liposomal membranes, reconstituted with actively pumping Na(+),K(+)-ATPase, in terms of nonequilibrium shape fluctuations that contain a relaxation time, τ, which is robust and independent of the specific fluctuation modes of the membrane. In the case of pumping Na(+)-ions, analysis of the flicker-noise temporal correlation spectrum of the liposomes leads to τ ~/= 0.5 s, comparing favorably with an intrinsic reaction-cycle time of about 0.4 s from enzymology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Na,K-ATPase reconstituted in liposomes: effects of lipid composition on hydrolytic activity and enzyme orientation.Colloids Surf B Biointerfaces. 2005 Apr 10;41(4):239-48. doi: 10.1016/j.colsurfb.2004.12.013. Colloids Surf B Biointerfaces. 2005. PMID: 15748819
-
Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics.Biochemistry. 2001 Jul 31;40(30):8842-51. doi: 10.1021/bi010541g. Biochemistry. 2001. PMID: 11467945
-
Hydrophobic ion interaction on Na+ activation and dephosphorylation of reconstituted Na+,K(+)-ATPase.Biochim Biophys Acta. 1995 May 4;1235(2):183-96. doi: 10.1016/0005-2736(95)80004-y. Biochim Biophys Acta. 1995. PMID: 7756325
-
Na/K-ATPase as an oligomeric ensemble.Biochemistry (Mosc). 2001 Aug;66(8):821-31. doi: 10.1023/a:1011964832767. Biochemistry (Mosc). 2001. PMID: 11566051 Review.
-
Interaction of (Na+ + K+)-ATPase with artificial membranes. I. Formation and structure of (Na+ + K+)-ATPase-liposomes.Biochim Biophys Acta. 1985 Dec 9;822(3-4):319-34. doi: 10.1016/0304-4157(85)90013-9. Biochim Biophys Acta. 1985. PMID: 2998473 Review. No abstract available.
Cited by
-
Is the fluid mosaic (and the accompanying raft hypothesis) a suitable model to describe fundamental features of biological membranes? What may be missing?Front Plant Sci. 2013 Nov 13;4:457. doi: 10.3389/fpls.2013.00457. Front Plant Sci. 2013. PMID: 24312108 Free PMC article. Review.
-
Bioinspired membrane-based systems for a physical approach of cell organization and dynamics: usefulness and limitations.Interface Focus. 2015 Aug 6;5(4):20150038. doi: 10.1098/rsfs.2015.0038. Interface Focus. 2015. PMID: 26464792 Free PMC article. Review.
-
Direct Cytoskeleton Forces Cause Membrane Softening in Red Blood Cells.Biophys J. 2015 Jun 16;108(12):2794-806. doi: 10.1016/j.bpj.2015.05.005. Biophys J. 2015. PMID: 26083919 Free PMC article.
-
Nonequilibrium fluctuations of lipid membranes by the rotating motor protein F1F0-ATP synthase.Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):11291-11296. doi: 10.1073/pnas.1701207114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29073046 Free PMC article.
-
Coupled Response of Membrane Hydration with Oscillating Metabolism in Live Cells: An Alternative Way to Modulate Structural Aspects of Biological Membranes?Biomolecules. 2019 Nov 2;9(11):687. doi: 10.3390/biom9110687. Biomolecules. 2019. PMID: 31684090 Free PMC article.
References
-
- Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438(7068):578–580. - PubMed
-
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. - PubMed
-
- Brochard F, Lennon J. Frequency spectrum of the flicker phenomenon in erythrocytes. J Phys. 1975;36:1035–1047.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

