Obesity-programmed mice are rescued by early genetic intervention
- PMID: 23093774
- PMCID: PMC3484438
- DOI: 10.1172/JCI62543
Obesity-programmed mice are rescued by early genetic intervention
Abstract
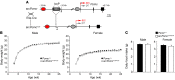
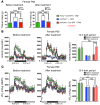
Obesity is a chronic metabolic disorder affecting half a billion people worldwide. Major difficulties in managing obesity are the cessation of continued weight loss in patients after an initial period of responsiveness and rebound to pretreatment weight. It is conceivable that chronic weight gain unrelated to physiological needs induces an allostatic regulatory state that defends a supranormal adipose mass despite its maladaptive consequences. To challenge this hypothesis, we generated a reversible genetic mouse model of early-onset hyperphagia and severe obesity by selectively blocking the expression of the proopiomelanocortin gene (Pomc) in hypothalamic neurons. Eutopic reactivation of central POMC transmission at different stages of overweight progression normalized or greatly reduced food intake in these obesity-programmed mice. Hypothalamic Pomc rescue also attenuated comorbidities such as hyperglycemia, hyperinsulinemia, and hepatic steatosis and normalized locomotor activity. However, effectiveness of treatment to normalize body weight and adiposity declined progressively as the level of obesity at the time of Pomc induction increased. Thus, our study using a novel reversible monogenic obesity model reveals the critical importance of early intervention for the prevention of subsequent allostatic overload that auto-perpetuates obesity.
Figures




Comment in
-
Tipping the scales early: probing the long-term effects of obesity.J Clin Invest. 2012 Nov;122(11):3840-2. doi: 10.1172/JCI66409. Epub 2012 Oct 24. J Clin Invest. 2012. PMID: 23093788 Free PMC article.
Similar articles
-
Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1861-70. doi: 10.1073/pnas.1500672112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825735 Free PMC article.
-
The homeodomain transcription factor Six3 regulates hypothalamic Pomc expression and its absence from POMC neurons induces hyperphagia and mild obesity in male mice.Mol Metab. 2024 Sep;87:101993. doi: 10.1016/j.molmet.2024.101993. Epub 2024 Jul 16. Mol Metab. 2024. PMID: 39025297 Free PMC article.
-
The transcriptional regulator PRDM12 is critical for Pomc expression in the mouse hypothalamus and controlling food intake, adiposity, and body weight.Mol Metab. 2020 Apr;34:43-53. doi: 10.1016/j.molmet.2020.01.007. Epub 2020 Jan 11. Mol Metab. 2020. PMID: 32180559 Free PMC article.
-
The neuroendocrine circuitry controlled by POMC, MSH, and AGRP.Handb Exp Pharmacol. 2012;(209):47-75. doi: 10.1007/978-3-642-24716-3_3. Handb Exp Pharmacol. 2012. PMID: 22249810 Review.
-
Molecular and functional genetics of the proopiomelanocortin gene, food intake regulation and obesity.FEBS Lett. 2017 Sep;591(17):2593-2606. doi: 10.1002/1873-3468.12776. Epub 2017 Aug 20. FEBS Lett. 2017. PMID: 28771698 Free PMC article. Review.
Cited by
-
The partial inhibition of hypothalamic IRX3 exacerbates obesity.EBioMedicine. 2019 Jan;39:448-460. doi: 10.1016/j.ebiom.2018.11.048. Epub 2018 Dec 3. EBioMedicine. 2019. PMID: 30522931 Free PMC article.
-
Reducing Adiposity in a Critical Developmental Window Has Lasting Benefits in Mice.Endocrinology. 2016 Feb;157(2):666-78. doi: 10.1210/en.2015-1753. Epub 2015 Nov 20. Endocrinology. 2016. PMID: 26587784 Free PMC article.
-
A Life without Hunger: The Ups (and Downs) to Modulating Melanocortin-3 Receptor Signaling.Front Neurosci. 2017 Mar 16;11:128. doi: 10.3389/fnins.2017.00128. eCollection 2017. Front Neurosci. 2017. PMID: 28360832 Free PMC article. Review.
-
Reprogramming the body weight set point by a reciprocal interaction of hypothalamic leptin sensitivity and Pomc gene expression reverts extreme obesity.Mol Metab. 2016 Aug 5;5(10):869-881. doi: 10.1016/j.molmet.2016.07.012. eCollection 2016 Oct. Mol Metab. 2016. PMID: 27689000 Free PMC article.
-
Role of POMC and AgRP neuronal activities on glycaemia in mice.Sci Rep. 2019 Sep 10;9(1):13068. doi: 10.1038/s41598-019-49295-7. Sci Rep. 2019. PMID: 31506541 Free PMC article.
References
-
- WHO . Factsheet no. 311: Obesity and Overweight. Geneva, Switzerland: World Health Organization; 2011.
-
- Pasquet P, Apfelbaum M. Recovery of initial body weight and composition after long-term massive overfeeding in men. Am J Clin Nutr. 1994;60(6):861–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

