Mechanism of growth inhibition of prostate cancer xenografts by valproic acid
- PMID: 23093837
- PMCID: PMC3471003
- DOI: 10.1155/2012/180363
Mechanism of growth inhibition of prostate cancer xenografts by valproic acid
Abstract
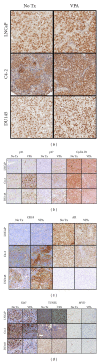
Valproic Acid (VPA), a histone deacetylase inhibitor, has been demonstrated to cause a marked decrease in proliferation of prostate cancer (PCa) cells in vitro and a significant reduction in tumor volume in vivo. The goal of this study is to better understand the VPA-induced growth inhibition in vivo, by studying expression of various markers in PCa xenografts.
Methods: For in vitro experiments, PCa cells were treated with 0, 0.6, and 1.2 mM VPA for 14 days. For in vivo models, experimental animals received 0.4% VPA in drinking water for 35 days. Tissue microarray was generated using cell pellets and excised xenografts.
Results: VPA treatment causes cell cycle arrest in PCa cells in vivo, as determined by increase in p21 and p27 and decrease in cyclin D1 expression. Increased expression of cytokeratin18 was also seen in xenografts. LNCaP xenografts in treated animals had reduced androgen receptor (AR) expression. While decreased proliferation was found in vitro, increase in apoptosis was found to be the reason for decreased tumor growth in vivo. Also, an anti-angiogenic effect was observed after VPA treatment.
Conclusion: VPA inhibits tumor growth by multiple mechanisms including cell cycle arrest, induction of differentiation, and inhibition of growth of tumor vasculature.
Figures

Similar articles
-
Multiple Molecular pathways explain the anti-proliferative effect of valproic acid on prostate cancer cells in vitro and in vivo.Prostate. 2007 Jul 1;67(10):1099-110. doi: 10.1002/pros.20587. Prostate. 2007. PMID: 17477369
-
Chronic administration of valproic acid inhibits PC3 cell growth by suppressing tumor angiogenesis in vivo.Int J Urol. 2007 Sep;14(9):838-45. doi: 10.1111/j.1442-2042.2007.01823.x. Int J Urol. 2007. PMID: 17760752
-
Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo.Cancer Res. 2006 Jul 15;66(14):7237-44. doi: 10.1158/0008-5472.CAN-05-0487. Cancer Res. 2006. PMID: 16849572
-
Low dosed interferon alpha augments the anti-tumor potential of histone deacetylase inhibition on prostate cancer cell growth and invasion.Prostate. 2012 Dec 1;72(16):1719-35. doi: 10.1002/pros.22525. Epub 2012 Apr 2. Prostate. 2012. PMID: 22473339
-
Rationale behind using valproic acid for Non-Hodgkin lymphoma: a biomolecular perspective.Eur Rev Med Pharmacol Sci. 2021 Dec;25(23):7486-7500. doi: 10.26355/eurrev_202112_27448. Eur Rev Med Pharmacol Sci. 2021. PMID: 34919251 Review.
Cited by
-
Valproic acid, an inhibitor of class I histone deacetylases, reverses acquired Erlotinib-resistance of lung adenocarcinoma cells: a Connectivity Mapping analysis and an experimental study.Am J Cancer Res. 2015 Jun 15;5(7):2202-11. eCollection 2015. Am J Cancer Res. 2015. PMID: 26328250 Free PMC article.
-
Histone deacetylases as targets for treatment of multiple diseases.Clin Sci (Lond). 2013 Jun;124(11):651-62. doi: 10.1042/CS20120504. Clin Sci (Lond). 2013. PMID: 23414309 Free PMC article. Review.
-
Valproic acid and its inhibition of tumor growth in systemic malignancies: beyond gliomas.J Neurooncol. 2013 Jul;113(3):531. doi: 10.1007/s11060-013-1129-z. Epub 2013 Apr 16. J Neurooncol. 2013. PMID: 23589035 No abstract available.
-
From HDAC to Voltage-Gated Ion Channels: What's Next? The Long Road of Antiepileptic Drugs Repositioning in Cancer.Cancers (Basel). 2022 Sep 10;14(18):4401. doi: 10.3390/cancers14184401. Cancers (Basel). 2022. PMID: 36139561 Free PMC article. Review.
-
Valproic Acid Inhibits NA-K-2CL Cotransporter RNA Expression in Male But Not in Female Rat Thymocytes.Dose Response. 2019 May 30;17(2):1559325819852444. doi: 10.1177/1559325819852444. eCollection 2019 Apr-Jun. Dose Response. 2019. PMID: 31210756 Free PMC article.
References
-
- Rephaeli A, Blank-Porat D, Tarasenko N, et al. In vivo and in vitro antitumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, in human prostate cancer. International Journal of Cancer. 2005;116(2):226–235. - PubMed
-
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Focus on acetylation: the role of histone deacetylase inhibitors in cancer therapy and beyond. Expert Opinion on Investigational Drugs. 2007;16(5):569–571. - PubMed
-
- Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. Journal of Cellular Biochemistry. 2005;96(2):293–304. - PubMed
-
- Sowa Y, Orita T, Hiranabe-Minamikawa S, et al. Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites. Annals of the New York Academy of Sciences. 1999;886:195–199. - PubMed
-
- Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle (Georgetown, Tex.) 2002;1(6):391–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
