Impact of regulatory requirements on medicine registration in African countries - perceptions and experiences of pharmaceutical companies in South Africa
- PMID: 23093897
- PMCID: PMC3471191
Impact of regulatory requirements on medicine registration in African countries - perceptions and experiences of pharmaceutical companies in South Africa
Abstract
Objective: Access to medicines has long been and remains a challenge in African countries. The impact of medicines registration policies in these countries poses a challenge for pharmaceutical companies wanting to register medicines in these countries. The recent AMRHI (African Medicines Registration Harmonisation Initiative) has increased the focus on the need for harmonisation. Medicines registration regulations differ across African countries. Anecdotal evidence, based on the experience of pharmaceutical companies on progress towards harmonisation is somewhat different, i.e. that country specific requirements were a barrier to the registration of medicines. The objective of this study was therefore to determine the nature and extent of regulatory hurdles experienced by pharmaceutical companies who wish to register and supply medicines to African countries.
Methods: This cross-sectional descriptive pilot study was conducted across pharmaceutical companies, both local and multinational. These companies were based in South Africa and were also members of Pharmaceutical Industry Association of South Africa (PIASA). The pharmaceutical companies supply both the private and public sectors. An online survey was developed using Survey Monkey. Survey questions focused on the following strands: nature and level of current supply of medicines to African countries by companies, general regulatory requirements, region specific questions and country specific questions across four regional economic communities in Africa, namely; Southern African Development Community (SADC), East African Community (EAC), Economic Community of the West African States (ECOWAS) and Economic Community of Central African States (ECCAS).
Results: A total of 33 responses were received to the questionnaire of which 26 respondents were from the PIASA Regulatory working group and 7 were from the PIASA Export working group.It was noted that since most of the regulatory authorities in Africa are resource-constrained, harmonisation of medicine registration policies will contribute positively to ensuring the safety, quality and efficacy of medicines. The experience of pharmaceutical companies indicated that country specific regulatory requirements are a barrier to registering and supplying medicines to African countries. In particular, GMP inspections, GMP inspection fees and country specific labeling were cited as key problems.
Conclusion: Pharmaceutical companies operating in African markets are experiencing difficulties in complying with the technical requirements of individual African countries. Further research is required to provide a balanced perspective on the country specific regulatory requirements vs. the African Regulatory Harmonisation Initiative (AMRHI).
Keywords: Good Manufacturing Practice; labeling; medicine registration; pharmaceutical policy.
Figures
References
-
- WHO Drug Information. 1. Vol. 24. World Health Organization; 2010. Regulatory Harmonisation. Updating medicines regulatory systems in sub-Saharan African countries; pp. 6–20.
-
- Drugs for Neglected Diseases Initiative (DNDi). Registering new drugs: The African context. New tools for new times. 2010 http://www.policycures.org/downloads/DNDi_Registering_New_Drugs-The_Afri....
-
- Assessment of medicines regulatory systems in sub-Saharan African countries. An overview of findings from 26 assessment reports. WHO; 2010. http://apps.who.int/medicinedocs/en/m/abstract/Js17577en/
-
- Moran Mary, Strub-Wourgaft Nathalie, Guzman Javier, Boulet Pascale, Wu Lindsey, Pecoul Bernard. Registering new drugs for low-income countries: the African challenge. PLoS Med. 2011 Feb;8(2):e1000411–6. doi: 10.1371/journal.pmed.1000411. http://pubmedcentralcanada.ca/pmcc/articles/pmid/21408102. - DOI - PMC - PubMed
-
- Sigonda M N, Ambali A. The African Medicines Regulatory Harmonisation Initiative: Rationale and Benefits. Clin Pharm Ther. 2011;89:176–178. - PubMed
LinkOut - more resources
Full Text Sources

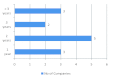
![Figure 2. Benefits of Harmonisation [adapted from reference 5, 8 & 10]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/a586/3471191/0f75cd867a0e/smr-05-031-g002.gif)