Probability fluxes and transition paths in a Markovian model describing complex subunit cooperativity in HCN2 channels
- PMID: 23093920
- PMCID: PMC3475657
- DOI: 10.1371/journal.pcbi.1002721
Probability fluxes and transition paths in a Markovian model describing complex subunit cooperativity in HCN2 channels
Abstract
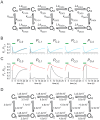
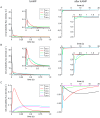
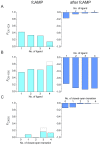
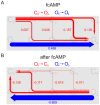
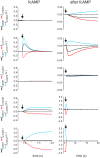
Hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels are voltage-gated tetrameric cation channels that generate electrical rhythmicity in neurons and cardiomyocytes. Activation can be enhanced by the binding of adenosine-3',5'-cyclic monophosphate (cAMP) to an intracellular cyclic nucleotide binding domain. Based on previously determined rate constants for a complex Markovian model describing the gating of homotetrameric HCN2 channels, we analyzed probability fluxes within this model, including unidirectional probability fluxes and the probability flux along transition paths. The time-dependent probability fluxes quantify the contributions of all 13 transitions of the model to channel activation. The binding of the first, third and fourth ligand evoked robust channel opening whereas the binding of the second ligand obstructed channel opening similar to the empty channel. Analysis of the net probability fluxes in terms of the transition path theory revealed pronounced hysteresis for channel activation and deactivation. These results provide quantitative insight into the complex interaction of the four structurally equal subunits, leading to non-equality in their function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Karplus M, McCammon JA (1981) The internal dynamics of globular proteins. CRC Crit Rev Biochem 9: 293–349. - PubMed
-
- Neher E, Sakmann B, Steinbach JH (1978) The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflug Arch Eur J Phy 375: 219–228. - PubMed
-
- Sigworth FJ, Neher E (1980) Single Na+ channel currents observed in cultured rat muscle cells. Nature 287: 447–449. - PubMed
-
- Conti F, Neher E (1980) Single channel recordings of K+ currents in squid axons. Nature 285: 140–143. - PubMed
-
- Markwick PR, McCammon JA (2011) Studying functional dynamics in bio-molecules using accelerated molecular dynamics. Phys Chem Chem Phys 13: 20053–20065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

