Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells
- PMID: 23094054
- PMCID: PMC3477155
- DOI: 10.1371/journal.pone.0047480
Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells
Abstract
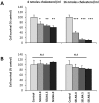
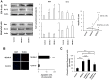
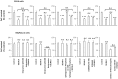
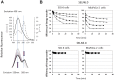
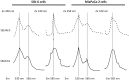
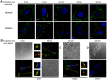
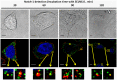
Exosomes are of increasing interest as alternative mode of cell-to-cell communication. We previously reported that exosomes secreted by human SOJ-6 pancreatic tumor cells induce (glyco)protein ligand-independent cell death and inhibit Notch-1 pathway, this latter being particularly active during carcinogenesis and in cancer stem cells. Therefore, we asked whether exosomal lipids were key-elements for cell death and hypothesized that cholesterol-rich membrane microdomains were privileged sites of exosome interactions with tumor cells. To address these questions and based on the lipid composition of exosomes from SOJ-6 cells (Ristorcelli et al. (2008) FASEB J. 22; 3358-3369) enriched in cholesterol and sphingomyelin (lipids forming liquid-ordered phase, Lo) and depleted in phospholipids (lipids forming liquid-disordered phase, Ld), we designed Synthetic Exosome-Like Nanoparticles (SELN) with ratios Lo/Ld from 3.0 to 6.0 framing that of SOJ-6 cell exosomes. SELN decreased tumor cell survival, the higher the Lo/Ld ratio, the lower the cell survival. This decreased survival was due to activation of cell death with inhibition of Notch pathway. FRET analyses indicated fusions/exchanges of SELN with cell membranes. Fluorescent SELN co-localized with the ganglioside GM1 then with Rab5A, markers of lipid microdomains and of early endosomes, respectively. These interactions occurred at lipid microdomains of plasma and/or endosome membranes where the Notch-1 pathway matures. We thus demonstrated a major role for lipids in interactions between SELN and tumor cells, and in the ensued cell death. To our knowledge this is the first report on such effects of lipidic nanoparticles on tumor cell behavior. This may have implications in tumor progression.
Conflict of interest statement
Figures




References
-
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G (2006) Exosomes: a common pathway for a specialized function. J Biochem 140: 13–21. - PubMed
-
- Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73: 1907–20. - PubMed
-
- Février B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages Curr Opin Cell Biol. 16: 415–421. - PubMed
-
- Nieuwland R, Sturk A (2010) Why do cells release vesicles? Thromb Res 125 Suppl 1 S49–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

