Cytokinesis in Drosophila male meiosis
- PMID: 23094234
- PMCID: PMC3469441
- DOI: 10.4161/spmg.21711
Cytokinesis in Drosophila male meiosis
Abstract
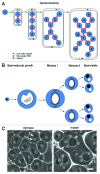
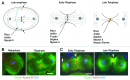
Cytokinesis separates the cytoplasm and the duplicated genome into two daughter cells at the end of cell division. This process must be finely regulated to maintain ploidy and prevent tumor formation. Drosophila male meiosis provides an excellent cell system for investigating cytokinesis. Mutants affecting this process can be easily identified and spermatocytes are large cells particularly suitable for cytological analysis of cytokinetic structures. Over the past decade, the powerful tools of Drosophila genetics and the unique characteristics of this cell system have led researchers to identify molecular players of the cell cleavage machinery and to address important open questions. Although spermatocyte cytokinesis is incomplete, resulting in formation of stable intercellular bridges, the molecular mechanisms are largely conserved in somatic cells. Thus, studies of Drosophila male meiosis will shed new light on the complex cell circuits regulating furrow ingression and substantially further our knowledge of cancer and other human diseases.
Figures
References
-
- Fuller MT. Spermatogenesis. In Bate M and Martinez-Arias A., eds. The Development of Drosophila melanogaster Cold Spring Harbor, NY: Cold Spring Harbor Press,1993:71-147.
-
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109:2779–88. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
