Palladium-catalyzed C-H functionalization using guanidine as a directing group: ortho arylation and olefination of arylguanidines
- PMID: 23095022
- PMCID: PMC3786205
- DOI: 10.1021/ol302533w
Palladium-catalyzed C-H functionalization using guanidine as a directing group: ortho arylation and olefination of arylguanidines
Abstract
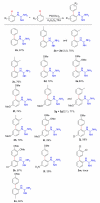
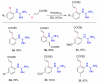
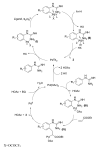
Palladium-catalyzed C-H functionalization using guanidine as the directing group was achieved under mild reaction conditions. Various guanidine derivatives were produced in moderate to good yields by using simple unactivated arenes or ethyl acrylate as the source of arylation or olefination, respectively.
Figures
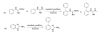
References
-
- Wang XS, Leow D, Yu JQ. J. Am. Chem. Soc. 2011;133:13864. - PMC - PubMed
- Zhao YS, He G, Nack WA, Chen G. Org. Lett. 2012;14:2948. - PubMed
- Yu M, Xie YG, Zhang YH. Org. Lett. 2012;14:2164. - PubMed
- Ji XC, Huang HW, Li YB, Chen HJ, Jiang HF. Angew. Chem., Int. Ed. 2012;51:1.
- Li DD, Yuan TT, Wang GW. ChemComm. 2011;47:12789. - PubMed
-
- Desai LV, Stower KJ, Sanford MS. J. Am. Chem. Soc. 2008;130:13285. - PMC - PubMed
- Chao F, Loh TP. ChemComm. 2011;47:10458.
- Ackermann L, Pospech J, Potukuchi HK. Org. Lett. 2012;14:2146. - PubMed
- Chen X, Li JJ, Hao XS, Goodhue CE, Yu JQ. J. Am. Chem. Soc. 2006;128:78. - PubMed
- Li DD, Yuan TT, Wang GW. J. Org. Chem. 2012;77:3341. - PubMed
- Thirunavukkarasu VS, Parthasarathy K, Cheng CH. Chem. Eur. J. 2010;16:1436. - PubMed
- Kishor P, Jeganmohan M. Org. Lett. 2011;13:6144. - PubMed
- Lu Y, Wang DH, Engle KM, Yu JQ. J. Am. Chem. Soc. 2010;132:5916. - PMC - PubMed
- Wang DH, Engle KM, Shi BF, Yu JQ. Science. 2010;327:315. - PMC - PubMed
- Shi BF, Zhang YH, Lam JK, Wang DH, Yu JQ. J. Am. Chem. Soc. 2010;132:460. - PMC - PubMed
-
- Igel P, Schneider E, Schnell D, Elz S, Seifert R, Buschauer A. J. Med. Chem. 2009;52:2623. - PubMed
-
- Coxon GD, Furman BL, Harvey AL, McTavish J, Mooney MH, Arastoo M, Kennedy AR, Tettey JM, Waigh RD. J. Med. Chem. 2009;52:3457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

