Terminal vs bridging hydrides of diiron dithiolates: protonation of Fe2(dithiolate)(CO)2(PMe3)4
- PMID: 23095145
- PMCID: PMC3518320
- DOI: 10.1021/ja3094394
Terminal vs bridging hydrides of diiron dithiolates: protonation of Fe2(dithiolate)(CO)2(PMe3)4
Abstract
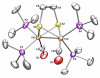
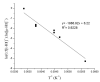
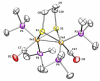
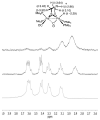
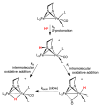
This investigation examines the protonation of diiron dithiolates, exploiting the new family of exceptionally electron-rich complexes Fe(2)(xdt)(CO)(2)(PMe(3))(4), where xdt is edt (ethanedithiolate, 1), pdt (propanedithiolate, 2), and adt (2-aza-1,3-propanedithiolate, 3), prepared by the photochemical substitution of the corresponding hexacarbonyls. Compounds 1-3 oxidize near -950 mV vs Fc(+/0). Crystallographic analyses confirm that 1 and 2 adopt C(2)-symmetric structures (Fe-Fe = 2.616 and 2.625 Å, respectively). Low-temperature protonation of 1 afforded exclusively [μ-H1](+), establishing the non-intermediacy of the terminal hydride ([t-H1](+)). At higher temperatures, protonation afforded mainly [t-H1](+). The temperature dependence of the ratio [t-H1](+)/[μ-H1](+) indicates that the barriers for the two protonation pathways differ by ∼4 kcal/mol. Low-temperature (31)P{(1)H} NMR measurements indicate that the protonation of 2 proceeds by an intermediate, proposed to be the S-protonated dithiolate [Fe(2)(Hpdt)(CO)(2)(PMe(3))(4)](+) ([S-H2](+)). This intermediate converts to [t-H2](+) and [μ-H2](+) by first-order and second-order processes, respectively. DFT calculations support transient protonation at sulfur and the proposal that the S-protonated species (e.g., [S-H2](+)) rearranges to the terminal hydride intramolecularly via a low-energy pathway. Protonation of 3 affords exclusively terminal hydrides, regardless of the acid or conditions, to give [t-H3](+), which isomerizes to [t-H3'](+), wherein all PMe(3) ligands are basal.
Figures





Similar articles
-
Isomerization of the hydride complexes [HFe2(SR)2(PR3)(x)(CO)(6-x)]+ (x = 2, 3, 4) relevant to the active site models for the [FeFe]-hydrogenases.Dalton Trans. 2010 Mar 28;39(12):3011-9. doi: 10.1039/b910147k. Epub 2009 Sep 16. Dalton Trans. 2010. PMID: 20221534 Free PMC article.
-
Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere.Acc Chem Res. 2015 Jul 21;48(7):2107-16. doi: 10.1021/acs.accounts.5b00177. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079848 Free PMC article.
-
New reactions of terminal hydrides on a diiron dithiolate.J Am Chem Soc. 2014 Apr 16;136(15):5773-82. doi: 10.1021/ja501366j. Epub 2014 Apr 8. J Am Chem Soc. 2014. PMID: 24661238
-
Excited state properties of diiron dithiolate hydrides: implications in the unsensitized photocatalysis of H2 evolution.Inorg Chem. 2013 Sep 3;52(17):9826-41. doi: 10.1021/ic400818t. Epub 2013 Aug 16. Inorg Chem. 2013. PMID: 23952259
-
DFT dissection of the reduction step in H2 catalytic production by [FeFe]-hydrogenase-inspired models: can the bridging hydride become more reactive than the terminal isomer?Inorg Chem. 2015 Oct 5;54(19):9529-42. doi: 10.1021/acs.inorgchem.5b01495. Epub 2015 Sep 11. Inorg Chem. 2015. PMID: 26359661
Cited by
-
Inhibition of [FeFe]-hydrogenase by formaldehyde: proposed mechanism and reactivity of FeFe alkyl complexes.Chem Sci. 2021 Nov 16;12(47):15673-15681. doi: 10.1039/d1sc05803g. eCollection 2021 Dec 8. Chem Sci. 2021. PMID: 35003598 Free PMC article.
-
Hydrogen Production Catalyzed by Bidirectional, Biomimetic Models of the [FeFe]-Hydrogenase Active Site.Organometallics. 2014 Oct 27;33(20):5897-5906. doi: 10.1021/om5004013. Epub 2014 Jul 1. Organometallics. 2014. PMID: 25364093 Free PMC article.
-
The maturase HydF enables [FeFe] hydrogenase assembly via transient, cofactor-dependent interactions.J Biol Chem. 2020 Aug 14;295(33):11891-11901. doi: 10.1074/jbc.RA119.011419. Epub 2020 Jul 3. J Biol Chem. 2020. PMID: 32620553 Free PMC article.
-
Computational investigation of [FeFe]-hydrogenase models: characterization of singly and doubly protonated intermediates and mechanistic insights.Inorg Chem. 2014 Oct 6;53(19):10301-11. doi: 10.1021/ic5013523. Epub 2014 Sep 10. Inorg Chem. 2014. PMID: 25207842 Free PMC article.
-
Protonation of nickel-iron hydrogenase models proceeds after isomerization at nickel.J Am Chem Soc. 2014 Sep 3;136(35):12385-95. doi: 10.1021/ja505783z. Epub 2014 Aug 21. J Am Chem Soc. 2014. PMID: 25094041 Free PMC article.
References
-
- Tard C, Pickett CJ. Chem. Rev. 2009;109:2245. - PubMed
-
- Silakov A, Wenk B, Reijerse E, Lubitz W. Phys. Chem. Chem. Phys. 2009;11:6592. - PubMed
-
- Nicolet Y, de Lacey AL, Vernede X, Fernandez VM, Hatchikian EC, Fontecilla-Camps JC. J. Am. Chem. Soc. 2001;123:1596. - PubMed
-
- Kramarz KW, Norton JR. Prog. Inorg. Chem. 1994;42:1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

