Directed evolution of bright mutants of an oxygen-independent flavin-binding fluorescent protein from Pseudomonas putida
- PMID: 23095243
- PMCID: PMC3488000
- DOI: 10.1186/1754-1611-6-20
Directed evolution of bright mutants of an oxygen-independent flavin-binding fluorescent protein from Pseudomonas putida
Abstract
Background: Fluorescent reporter proteins have revolutionized our understanding of cellular bioprocesses by enabling live cell imaging with exquisite spatio-temporal resolution. Existing fluorescent proteins are predominantly based on the green fluorescent protein (GFP) and related analogs. However, GFP-family proteins strictly require molecular oxygen for maturation of fluorescence, which precludes their application for investigating biological processes in low-oxygen environments. A new class of oxygen-independent fluorescent reporter proteins was recently reported based on flavin-binding photosensors from Bacillus subtilis and Pseudomonas putida. However, flavin-binding fluorescent proteins show very limited brightness, which restricts their utility as biological imaging probes.
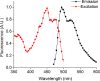
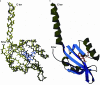
Results: In this work, we report the discovery of bright mutants of a flavin-binding fluorescent protein from P. putida using directed evolution by site saturation mutagenesis. We discovered two mutations at a chromophore-proximal amino acid (F37S and F37T) that confer a twofold enhancement in brightness relative to the wild type fluorescent protein through improvements in quantum yield and holoprotein fraction. In addition, we observed that substitution with other aromatic amino acids at this residue (F37Y and F37W) severely diminishes fluorescence emission. Therefore, we identify F37 as a key amino acid residue in determining fluorescence.
Conclusions: To increase the scope and utility of flavin-binding fluorescent proteins as practical fluorescent reporters, there is a strong need for improved variants of the wild type protein. Our work reports on the application of site saturation mutagenesis to isolate brighter variants of a flavin-binding fluorescent protein, which is a first-of-its-kind approach. Overall, we anticipate that the improved variants will find pervasive use as fluorescent reporters for biological studies in low-oxygen environments.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

