Strategy for eliciting antigen-specific CD8+ T cell-mediated immune response against a cryptic CTL epitope of merkel cell polyomavirus large T antigen
- PMID: 23095249
- PMCID: PMC3499220
- DOI: 10.1186/2045-3701-2-36
Strategy for eliciting antigen-specific CD8+ T cell-mediated immune response against a cryptic CTL epitope of merkel cell polyomavirus large T antigen
Abstract
Background: Merkel cell carcinoma (MCC) is a relatively new addition to the expanding category of oncovirus-induced cancers. Although still comparably rare, the number of cases has risen dramatically in recent years. Further complicating this trend is that MCC is an extremely aggressive neoplasm with poor patient prognosis and limited treatment options for advanced disease. The causative agent of MCC has been identified as the merkel cell polyomavirus (MCPyV). The MCPyV-encoded large T (LT) antigen is an oncoprotein that is theorized to be essential for virus-mediated tumorigenesis and is therefore, an excellent MCC antigen for the generation of antitumor immune responses. As a foreign antigen, the LT oncoprotein avoids the obstacle of immune tolerance, which normally impedes the development of antitumor immunity. Ergo, it is an excellent target for anti-MCC immunotherapy. Since tumor-specific CD8+ T cells lead to better prognosis for MCC and numerous other cancers, we have generated a DNA vaccine that is capable of eliciting LT-specific CD8+ T cells. The DNA vaccine (pcDNA3-CRT/LT) encodes the LT antigen linked to a damage-associated molecular pattern, calreticulin (CRT), as it has been demonstrated that the linkage of CRT to antigens promotes the induction of antigen-specific CD8+ T cells.
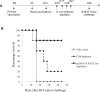
Results: The present study shows that DNA vaccine-induced generation of LT-specific CD8+ T cells is augmented by linking CRT to the LT antigen. This is relevant since the therapeutic effects of the pcDNA3-CRT/LT DNA vaccine is mediated by LT-specific CD8+ T cells. Mice vaccinated with the DNA vaccine produced demonstrably more LT-specific CD8+ T cells. The DNA vaccine was also able to confer LT-specific CD8+ T cell-mediated protective and therapeutic effects to prolong the survival of mice with LT-expressing tumors. In the interest of determining the LT epitope which most MCC-specific CD8+ T cells recognize, we identified the amino acid sequence of the immunodominant LT epitope as aa19-27 (IAPNCYGNI) and found that it is H-2kb-restricted.
Conclusion: The results of this study can facilitate the development of other modes of MCC treatment such as peptide-based vaccines and adoptive transfer of LT-specific CD8+ T cells. Likewise, the MCC DNA vaccine has great potential for clinical translation as the immunologic specificity is high and the treatment strategy can be exported to address other virus-induced tumors.
Figures





References
-
- Herbst A, Haynes HA, Nghiem P. The standard of care for Merkel cell carcinoma should include adjuvant radiation and lymph node surgery. J Am Acad Dermatol. 2002;46(4):640–642. Epub 2002/03/22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

