FBXW7 is involved in Aurora B degradation
- PMID: 23095493
- PMCID: PMC3507501
- DOI: 10.4161/cc.22381
FBXW7 is involved in Aurora B degradation
Abstract
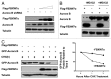
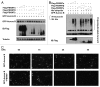
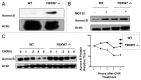
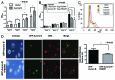
FBXW7, a component of E3 ubiquitin ligase, plays an important role in mitotic checkpoint, but its role remains unclear. Aurora B is a mitotic checkpoint kinase that plays a pivotal role in mitosis by ensuring correct chromosome segregation and normal progression through mitosis. Whether Aurora B and FBXW7 are coordinately regulated during mitosis is not known. Here, we show that FBXW7 is a negative regulator for Aurora B. Ectopic expression of FBXW7 can suppress the expression of Aurora B. Accordingly, FBXW7 deficiency leads to Aurora B elevation. Mechanistic studies show that all FBXW7 isoforms are negative regulators of Aurora B expression through ubiquitination-mediated protein degradation. Aurora B interacts with R465 and R505 residues of WD 40 domain of FBXW7. Significantly, inverse correlation between FBXW7 and Aurora B elevation is translated into the deregulation of mitosis. FBWX7 expression mitigates Aurora B-mediated cell growth and mitotic deregulation. In addition, FBXW7 reduces the percentage of multinucleated cells caused by Aurora B overexpression. These data suggest that FBXW7 is an important negative regulator of Aurora B, and that the loss or mutation of FBXW7 as seen in many types of cancer could lead to an abnormal elevation of Aurora B and result in deregulated mitosis, which accelerates cancer cell growth.
Figures


Comment in
-
FBW7-Aurora B-p53 feedback loop regulates mitosis and cell growth.Cell Cycle. 2012 Nov 15;11(22):4113-4. doi: 10.4161/cc.22607. Epub 2012 Oct 25. Cell Cycle. 2012. PMID: 23099923 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
