Differential effects of FK506 on structural and functional axonal deficits after diffuse brain injury in the immature rat
- PMID: 23095847
- PMCID: PMC3495060
- DOI: 10.1097/NEN.0b013e31826f5876
Differential effects of FK506 on structural and functional axonal deficits after diffuse brain injury in the immature rat
Abstract
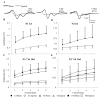
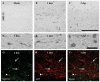
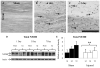
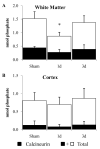
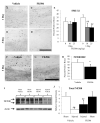
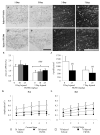
Diffuse axonal injury is a major component of traumatic brain injury in children and correlates with long-term cognitive impairment. Traumatic brain injury in adult rodents has been linked to a decrease in compound action potential (CAP) in the corpus callosum, but information on trauma-associated diffuse axonal injury in immature rodents is limited. We investigated the effects of closed head injury on CAP in the corpus callosum of 17-day-old rats. The injury resulted in CAP deficits of both myelinated and unmyelinated fibers in the corpus callosum between 1 and 14 days postinjury (dpi). These deficits were accompanied by intra-axonal dephosphorylation of the 200-kDa neurofilament subunit (NF200) at 1 and 3 dpi, a decrease in total NF200 at 3 dpi and axonal degeneration at 3 and 7 dpi. Although total phosphatase activity decreased at 1 dpi, calcineurin activity was unchanged. The calcineurin inhibitor, FK506, significantly attenuated the injury-induced NF200 dephosphorylation of NF200 at 3 dpi and axonal degeneration at 3 and 7 dpi but did not affect the decrease in NF200 protein levels or impaired axonal transport. FK506 had no effect on CAP deficits at 3 dpi but exacerbated the deficit in only the myelinated fibers at 7 dpi. Thus, in contrast to adult animals, FK506 treatment did not improve axonal function in brain-injured immature animals, suggesting that calcineurin may not contribute to impaired axonal function.
Figures
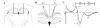

Similar articles
-
Preferential neuroprotective effect of tacrolimus (FK506) on unmyelinated axons following traumatic brain injury.Brain Res. 2007 Jun 18;1154:225-36. doi: 10.1016/j.brainres.2007.04.002. Epub 2007 Apr 5. Brain Res. 2007. PMID: 17481596 Free PMC article.
-
The effects of cyclosporin-A on axonal conduction deficits following traumatic brain injury in adult rats.Exp Neurol. 2010 Jul;224(1):241-51. doi: 10.1016/j.expneurol.2010.03.026. Epub 2010 Apr 1. Exp Neurol. 2010. PMID: 20362574 Free PMC article.
-
Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury.Exp Neurol. 2005 Nov;196(1):126-37. doi: 10.1016/j.expneurol.2005.07.014. Epub 2005 Aug 18. Exp Neurol. 2005. PMID: 16109409
-
The immunophilin ligand FK506 attenuates the axonal damage associated with rapid rewarming following posttraumatic hypothermia.Exp Neurol. 2001 Nov;172(1):199-210. doi: 10.1006/exnr.2001.7765. Exp Neurol. 2001. PMID: 11681852
-
Pathobiology of traumatically induced axonal injury in animals and man.Ann Emerg Med. 1993 Jun;22(6):980-6. doi: 10.1016/s0196-0644(05)82738-6. Ann Emerg Med. 1993. PMID: 8503536 Review.
Cited by
-
Neurotrophic Effects of Mu Bie Zi (Momordica cochinchinensis) Seed Elucidated by High-Throughput Screening of Natural Products for NGF Mimetic Effects in PC-12 Cells.Neurochem Res. 2015 Oct;40(10):2102-12. doi: 10.1007/s11064-015-1560-y. Epub 2015 Apr 11. Neurochem Res. 2015. PMID: 25862192 Free PMC article.
-
Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum.J Neuropathol Exp Neurol. 2013 Dec;72(12):1106-25. doi: 10.1097/NEN.0000000000000009. J Neuropathol Exp Neurol. 2013. PMID: 24226267 Free PMC article.
-
Acute axon damage and demyelination are mitigated by 4-aminopyridine (4-AP) therapy after experimental traumatic brain injury.Acta Neuropathol Commun. 2022 May 2;10(1):67. doi: 10.1186/s40478-022-01366-z. Acta Neuropathol Commun. 2022. PMID: 35501931 Free PMC article.
-
Stem Cell Therapy for Pediatric Traumatic Brain Injury.Front Neurol. 2020 Dec 2;11:601286. doi: 10.3389/fneur.2020.601286. eCollection 2020. Front Neurol. 2020. PMID: 33343501 Free PMC article. Review.
-
Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments.Front Cell Neurosci. 2014 Dec 17;8:429. doi: 10.3389/fncel.2014.00429. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25565963 Free PMC article. Review.
References
-
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–38. - PubMed
-
- Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60:1–32. - PubMed
-
- Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56:36–50. - PubMed
-
- Babikian T, Freier MC, Tong KA, et al. Susceptibility weighted imaging: neuropsychologic outcome and pediatric head injury. Pediatr Neurol. 2005;33:184–94. - PubMed
-
- Babikian T, Tong KA, Galloway NR, et al. Diffusion-weighted imaging predicts cognition in pediatric brain injury. Pediatr Neurol. 2009;41:406–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

