Development of new drugs for an old target: the penicillin binding proteins
- PMID: 23095893
- PMCID: PMC6268044
- DOI: 10.3390/molecules171112478
Development of new drugs for an old target: the penicillin binding proteins
Abstract
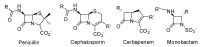
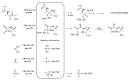
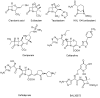
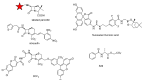
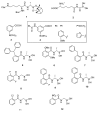
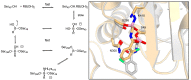
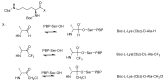
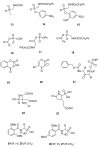
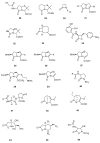
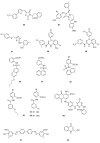
The widespread use of β-lactam antibiotics has led to the worldwide appearance of drug-resistant strains. Bacteria have developed resistance to β-lactams by two main mechanisms: the production of β-lactamases, sometimes accompanied by a decrease of outer membrane permeability, and the production of low-affinity, drug resistant Penicillin Binding Proteins (PBPs). PBPs remain attractive targets for developing new antibiotic agents because they catalyse the last steps of the biosynthesis of peptidoglycan, which is unique to bacteria, and lies outside the cytoplasmic membrane. Here we summarize the “current state of the art” of non-β-lactam inhibitors of PBPs, which have being developed in an attempt to counter the emergence of β-lactam resistance. These molecules are not susceptible to hydrolysis by β-lactamases and thus present a real alternative to β-lactams. We present transition state analogs such as boronic acids, which can covalently bind to the active serine residue in the catalytic site. Molecules containing ring structures different from the β-lactam-ring like lactivicin are able to acylate the active serine residue. High throughput screening methods, in combination with virtual screening methods and structure based design, have allowed the development of new molecules. Some of these novel inhibitors are active against major pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and thus open avenues new for the discovery of novel antibiotics.
Figures
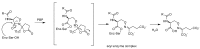
References
-
- Rammelkamp C.H., Maxon T. Resistance of Staphylococcus aureus to the Action of Penicillin. Proc. Soc. Exp. Biol. Med. 1942;51:386–389.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

