Three-dimensional cultures of mouse mammary epithelial cells
- PMID: 23097110
- PMCID: PMC3666564
- DOI: 10.1007/978-1-62703-125-7_14
Three-dimensional cultures of mouse mammary epithelial cells
Abstract
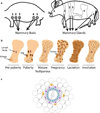
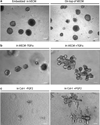
The mammary gland is an ideal "model organism" for studying tissue specificity and gene expression in mammals: it is one of the few organs that develop after birth and it undergoes multiple cycles of growth, differentiation and regression during the animal's lifetime in preparation for the important function of lactation. The basic "functional differentiation" unit in the gland is the mammary acinus made up of a layer of polarized epithelial cells specialized for milk production surrounded by myoepithelial contractile cells, and the two-layered structure is surrounded by basement membrane. Much knowledge about the regulation of mammary gland development has been acquired from studying the physiology of the gland and of lactation in rodents. Culture studies, however, were hampered by the inability to maintain functional differentiation on conventional tissue culture plastic. We now know that the microenvironment, including the extracellular matrix and tissue architecture, plays a crucial role in directing functional differentiation of organs. Thus, in order for culture systems to be effective experimental models, they need to recapitulate the basic unit of differentiated function in the tissue or organ and to maintain its three-dimensional (3D) structure. Mouse mammary culture models evolved from basic monolayers of cells to an array of complex 3D systems that observe the importance of the microenvironment in dictating proper tissue function and structure. In this chapter, we focus on how 3D mouse mammary epithelial cultures have enabled investigators to gain a better understanding of the organization, development and function of the acinus, and to identify key molecular, structural, and mechanical cues important for maintaining mammary function and architecture. The accompanying chapter of Vidi et al. describes 3D models developed for human cells. Here, we describe how mouse primary epithelial cells and cell lines--essentially those we use in our laboratory--are cultured in relevant 3D microenvironments. We focus on the design of functional assays that enable us to understand the intricate signaling events underlying mammary gland biology, and address the advantages and limitations of the different culture settings. Finally we also discuss how advances in bioengineering tools may help towards the ultimate goal of building tissues and organs in culture for basic research and clinical studies.
Figures
Similar articles
-
Mammary gland 3D cell culture systems in farm animals.Vet Res. 2021 Jun 2;52(1):78. doi: 10.1186/s13567-021-00947-5. Vet Res. 2021. PMID: 34078471 Free PMC article. Review.
-
Using 3D Culture of Primary Mammary Epithelial Cells to Define Molecular Entities Required for Acinus Formation: Analyzing MAP Kinase Phosphatases.Methods Mol Biol. 2017;1501:199-216. doi: 10.1007/978-1-4939-6475-8_9. Methods Mol Biol. 2017. PMID: 27796954
-
Derivation of a robust mouse mammary organoid system for studying tissue dynamics.Development. 2017 Mar 15;144(6):1065-1071. doi: 10.1242/dev.145045. Epub 2016 Dec 19. Development. 2017. PMID: 27993977
-
Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation.J Mammary Gland Biol Neoplasia. 2012 Jun;17(2):103-10. doi: 10.1007/s10911-012-9251-7. Epub 2012 May 10. J Mammary Gland Biol Neoplasia. 2012. PMID: 22573197 Review.
-
Matrix-based three-dimensional culture of buffalo mammary epithelial cells showed higher induction of genes related to milk protein and fatty acid metabolism.Cell Biol Int. 2016 Feb;40(2):232-8. doi: 10.1002/cbin.10555. Epub 2015 Nov 15. Cell Biol Int. 2016. PMID: 26503422
Cited by
-
Characterization of primary human mammary epithelial cells isolated and propagated by conditional reprogrammed cell culture.Oncotarget. 2017 Dec 22;9(14):11503-11514. doi: 10.18632/oncotarget.23817. eCollection 2018 Feb 20. Oncotarget. 2017. PMID: 29545915 Free PMC article.
-
A miR-125/Sirtuin-7 pathway drives the pro-calcific potential of myeloid cells in diabetic vascular disease.Diabetologia. 2022 Sep;65(9):1555-1568. doi: 10.1007/s00125-022-05733-2. Epub 2022 Jun 16. Diabetologia. 2022. PMID: 35708762 Free PMC article.
-
The Role of Microfluidics for Organ on Chip Simulations.Bioengineering (Basel). 2017 May 4;4(2):39. doi: 10.3390/bioengineering4020039. Bioengineering (Basel). 2017. PMID: 28952518 Free PMC article. Review.
-
Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions.Matrix Biol. 2017 Jan;57-58:324-333. doi: 10.1016/j.matbio.2016.06.002. Epub 2016 Jun 6. Matrix Biol. 2017. PMID: 27283894 Free PMC article. Review.
-
The atypical cyclin-like protein Spy1 overrides p53-mediated tumour suppression and promotes susceptibility to breast tumourigenesis.Breast Cancer Res. 2019 Dec 11;21(1):140. doi: 10.1186/s13058-019-1211-3. Breast Cancer Res. 2019. PMID: 31829284 Free PMC article.
References
-
- Babinet C. Transgenic mice: an irreplaceable tool for the study of mammalian development and biology. J Am Soc Nephrol. 2000;11(Suppl 16):S88–S94. - PubMed
-
- Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1:206–214. - PubMed
-
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. - PubMed
-
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

