The host proteins transportin SR2/TNPO3 and cyclophilin A exert opposing effects on HIV-1 uncoating
- PMID: 23097435
- PMCID: PMC3536424
- DOI: 10.1128/JVI.07177-11
The host proteins transportin SR2/TNPO3 and cyclophilin A exert opposing effects on HIV-1 uncoating
Abstract
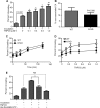
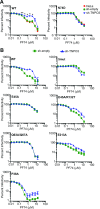
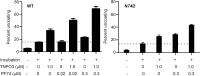
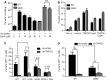
Following entry of the HIV-1 core into target cells, productive infection depends on the proper disassembly of the viral capsid (uncoating). Although much is known regarding HIV-1 entry, the actions of host cell proteins that HIV-1 utilizes during early postentry steps are poorly understood. One such factor, transportin SR2 (TRN-SR2)/transportin 3 (TNPO3), promotes infection by HIV-1 and some other lentiviruses, and recent studies have genetically linked TNPO3 dependence of infection to the viral capsid protein (CA). Here we report that purified recombinant TNPO3 stimulates the uncoating of HIV-1 cores in vitro. The stimulatory effect was reduced by RanGTP, a known ligand for transportin family members. Depletion of TNPO3 in target cells rendered HIV-1 less susceptible to inhibition by PF74, a small-molecule HIV-1 inhibitor that induces premature uncoating. In contrast to the case for TNPO3, addition of the CA-binding host protein cyclophilin A (CypA) inhibited HIV-1 uncoating and reduced the stimulatory effect of TNPO3 on uncoating in vitro. In cells in which TNPO3 was depleted, HIV-1 infection was enhanced 4-fold by addition of cyclosporine, indicating that the requirement for TNPO3 in HIV-1 infection is modulated by CypA-CA interactions. Although TNPO3 was localized primarily to the cytoplasm, depletion of TNPO3 from target cells inhibited HIV-1 infection without reducing the accumulation of nuclear proviral DNA, suggesting that TNPO3 facilitates a stage of the virus life cycle subsequent to nuclear entry. Our results suggest that TNPO3 and cyclophilin A facilitate HIV-1 infection by coordinating proper uncoating of the core in target cells.
Figures


Similar articles
-
Role of Transportin-SR2 in HIV-1 Nuclear Import.Viruses. 2021 May 4;13(5):829. doi: 10.3390/v13050829. Viruses. 2021. PMID: 34064404 Free PMC article. Review.
-
Interplay between HIV entry and transportin-SR2 dependency.Retrovirology. 2011 Jan 30;8:7. doi: 10.1186/1742-4690-8-7. Retrovirology. 2011. PMID: 21276267 Free PMC article.
-
Transportin-1 binds to the HIV-1 capsid via a nuclear localization signal and triggers uncoating.Nat Microbiol. 2019 Nov;4(11):1840-1850. doi: 10.1038/s41564-019-0575-6. Epub 2019 Oct 14. Nat Microbiol. 2019. PMID: 31611641
-
Daxx Inhibits HIV-1 Reverse Transcription and Uncoating in a SUMO-Dependent Manner.Viruses. 2020 Jun 11;12(6):636. doi: 10.3390/v12060636. Viruses. 2020. PMID: 32545337 Free PMC article.
-
A model for cofactor use during HIV-1 reverse transcription and nuclear entry.Curr Opin Virol. 2014 Feb;4(100):32-6. doi: 10.1016/j.coviro.2013.11.003. Epub 2014 Jan 14. Curr Opin Virol. 2014. PMID: 24525292 Free PMC article. Review.
Cited by
-
Nuclear import of APOBEC3F-labeled HIV-1 preintegration complexes.Proc Natl Acad Sci U S A. 2013 Dec 3;110(49):E4780-9. doi: 10.1073/pnas.1315996110. Epub 2013 Nov 18. Proc Natl Acad Sci U S A. 2013. PMID: 24248339 Free PMC article.
-
The HIV-1 integrase mutant R263A/K264A is 2-fold defective for TRN-SR2 binding and viral nuclear import.J Biol Chem. 2014 Sep 5;289(36):25351-61. doi: 10.1074/jbc.M113.533281. Epub 2014 Jul 25. J Biol Chem. 2014. PMID: 25063804 Free PMC article.
-
Physical properties of the HIV-1 capsid from all-atom molecular dynamics simulations.Nat Commun. 2017 Jul 19;8:15959. doi: 10.1038/ncomms15959. Nat Commun. 2017. PMID: 28722007 Free PMC article.
-
Degradation of SAMHD1 by Vpx Is Independent of Uncoating.J Virol. 2015 May;89(10):5701-13. doi: 10.1128/JVI.03575-14. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762741 Free PMC article.
-
Interactions of HIV-1 Capsid with Host Factors and Their Implications for Developing Novel Therapeutics.Viruses. 2021 Mar 5;13(3):417. doi: 10.3390/v13030417. Viruses. 2021. PMID: 33807824 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

