LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice
- PMID: 23097495
- PMCID: PMC3521678
- DOI: 10.1091/mbc.E12-09-0673
LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice
Abstract
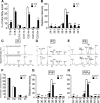
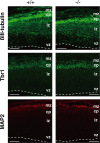
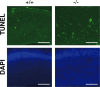
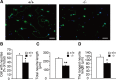
Dietary arachidonic acid (AA) has roles in growth, neuronal development, and cognitive function in infants. AA is remarkably enriched in phosphatidylinositol (PI), an important constituent of biological membranes in mammals; however, the physiological significance of AA-containing PI remains unknown. In an RNA interference-based genetic screen using Caenorhabditis elegans, we recently cloned mboa-7 as an acyltransferase that selectively incorporates AA into PI. Here we show that lysophosphatidylinositol acyltransferase 1 (LPIAT1, also known as MBOAT7), the closest mammalian homologue, plays a crucial role in brain development in mice. Lpiat1(-/-) mice show almost no LPIAT activity with arachidonoyl-CoA as an acyl donor and show reduced AA contents in PI and PI phosphates. Lpiat1(-/-) mice die within a month and show atrophy of the cerebral cortex and hippocampus. Immunohistochemical analysis reveals disordered cortical lamination and delayed neuronal migration in the cortex of E18.5 Lpiat1(-/-) mice. LPIAT1 deficiency also causes disordered neuronal processes in the cortex and reduced neurite outgrowth in vitro. Taken together, these results demonstrate that AA-containing PI/PI phosphates play an important role in normal cortical lamination during brain development in mice.
Figures





References
-
- Abad-Jorge A. The role of DHA and ARA in infant nutrition and neurodevelopmental outcomes. Today's Dietician. 2008;10:66.
-
- Backer JM. The regulation and function of class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. - PubMed
-
- Baker RR, Thompson W. Selective acylation of 1-acylglycerophosphorylinositol by rat brain microsomes. Comparison with 1-acylglycerophosphorylcholine. J Biol Chem. 1973;248:7060–7065. - PubMed
-
- Bannai H, Inoue T, Nakayama T, Hattori M, Mikoshiba K. Kinesin dependent, rapid, bi-directional transport of ER sub-compartment in dendrites of hippocampal neurons. J Cell Sci. 2004;117:163–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

