Molecules affecting hypothalamic control of core body temperature in response to calorie intake
- PMID: 23097647
- PMCID: PMC3466567
- DOI: 10.3389/fgene.2012.00184
Molecules affecting hypothalamic control of core body temperature in response to calorie intake
Abstract
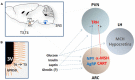
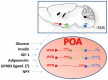
Core body temperature (CBT) and calorie intake are main components of energy homeostasis and two important regulators of health, longevity, and aging. In homeotherms, CBT can be influenced by calorie intake as food deprivation or calorie restriction (CR) lowers CBT whereas feeding has hyperthermic effects. The finding that in mice CBT prolonged lifespan independently of CR, suggested that the mechanisms modulating CBT may represent important regulators of aging. Here we summarize the current knowledge on the signaling molecules and their receptors that participate in the regulation of CBT responses to calorie intake. These include hypothalamic neuropeptides regulating feeding but also energy expenditure via modulation of thermogenesis. We also report studies indicating that nutrient signals can contribute to regulation of CBT by direct action on hypothalamic preoptic warm-sensitive neurons that in turn regulate adaptive thermogenesis and hence CBT. Finally, we show the role played by two orphans G protein-coupled receptor: GPR50 and GPR83, that were recently demonstrated to regulate temperature-dependent energy expenditure.
Keywords: GPCR; calorie restriction; core body temperature; homeostasis; hypothalamus; neuropeptides; warm-sensitive neurons.
Figures
References
-
- Adachi A., Shimizu N., Oomura Y., Kobashi M. (1984). Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci. Lett. 46 215–218 - PubMed
-
- Adams F., Grassie M., Shahid M., Hill D. R., Henry B. (2003). Acute oral dexamethasone administration reduces levels of orphan GPCR glucocorticoid-induced receptor (GIR) mRNA in rodent brain: potential role in HPA-axis function. Brain Res. Mol. Brain Res. 117 39–46 - PubMed
-
- Ahima R. S. (2000). Leptin and the neuroendocrinology of fasting. Front. Horm. Res. 26 42–56 - PubMed
-
- Ahima R. S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., et al. (1996). Role of leptin in the neuroendocrine response to fasting. Nature 382 250–252 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

