Structural and kinetic effects on changes in the CO(2) binding pocket of human carbonic anhydrase II
- PMID: 23098192
- PMCID: PMC4301431
- DOI: 10.1021/bi301155z
Structural and kinetic effects on changes in the CO(2) binding pocket of human carbonic anhydrase II
Abstract
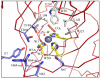
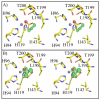
This work examines the effect of perturbing the position of bound CO(2) in the active site of human carbonic anhydrase II (HCA II) on catalysis. Variants of HCA II in which Val143 was replaced with hydrophobic residues Ile, Leu, and Ala were examined. The efficiency of catalysis in the hydration of CO(2) for these variants was characterized by (18)O exchange mass spectrometry, and their structures were determined by X-ray crystallography at 1.7-1.5 Å resolution. The most hydrophobic substitutions, V143I and V143L, showed decreases in the level of catalysis, as much as 20-fold, while the replacement by the smaller V143A mutation showed an only moderate 2-fold decrease in activity. Structural data for all three variants show no significant change in the overall position of amino acid side chains in the active site compared with the wild type. However, V143A HCA II showed additional ordered water molecules in the active site compared to the number for the wild type. To further investigate the decrease in the catalytic efficiency of V143I HCA II, an X-ray crystallographic CO(2) entrapment experiment was performed to 0.93 Å resolution. This structure revealed an unexpected shift in the CO(2) substrate toward the zinc-bound solvent, placing it ~0.3 Ǻ closer than previously observed in the wild type in conjunction with the observed dual occupancy of the product bicarbonate, presumably formed during the acquisition of data. These data suggest that the Ile substitution at position 143 reduced the catalytic efficiency, which is likely due to steric crowding resulting in destabilization of the transition state for conversion of CO(2) into bicarbonate and a decreased product dissociation rate.
Figures





References
-
- Silverman DN, McKenna R. Solvent-Mediated Proton Transfer in Catalysis by Carbonic Anhydrase. Acc Chem Res. 2007;40:669–675. - PubMed
-
- Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74:1–20. - PubMed
-
- Christianson DW, Fierke CA. Carbonic anhydrase: Evolution of the zinc binding site by nature and by design. Accounts of Chemical Research. 1996;29:331–339.
-
- Supuran CT, Scozzafava A, Conway J. Carbonic Anhydrase - Its Inhibitors and Activators. CRC Press; Boca Raton: 2004.
-
- Chegwidden WR, Carter ND, Edwards YH. The Carbonic Anhydrases New Horizons. Birkhauser Verlag; Basel: 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

