A versatile monoclonal antibody specific to human SERPINB5
- PMID: 23098299
- PMCID: PMC3482377
- DOI: 10.1089/hyb.2012.0035
A versatile monoclonal antibody specific to human SERPINB5
Abstract
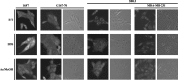
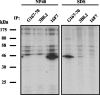
Maspin (SERPINB5) is a member of the Clade B subgroup of the large superfamily of serine protease inhibitors. It is proposed that maspin is a tumor suppressor; however, its molecular role remains to be elucidated. Here we report the characterization of a mouse monoclonal antibody directed against human maspin. This antibody, 16F7, recognizes maspin in both its native and denatured form, unlike several other commercial antibodies tested in this study. It will be a useful and versatile tool for future analyses of the biological function of maspin.
Figures


References
-
- Silverman GA. Whisstock JC. Askew DJ. Pak SC. Luke CJ. Cataltepe S. Irving JA. Bird PI. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci. 2004;61:301–325. - PMC - PubMed
-
- Irving JA. Pike RN. Lesk AM. Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

