Mechanisms underlying ICU muscle wasting and effects of passive mechanical loading
- PMID: 23098317
- PMCID: PMC3682313
- DOI: 10.1186/cc11841
Mechanisms underlying ICU muscle wasting and effects of passive mechanical loading
Abstract
Introduction: Critically ill ICU patients commonly develop severe muscle wasting and impaired muscle function, leading to delayed recovery, with subsequent increased morbidity and financial costs, and decreased quality of life for survivors. Critical illness myopathy (CIM) is a frequently observed neuromuscular disorder in ICU patients. Sepsis, systemic corticosteroid hormone treatment and post-synaptic neuromuscular blockade have been forwarded as the dominating triggering factors. Recent experimental results from our group using a unique experimental rat ICU model show that the mechanical silencing associated with CIM is the primary triggering factor. This study aims to unravel the mechanisms underlying CIM, and to evaluate the effects of a specific intervention aiming at reducing mechanical silencing in sedated and mechanically ventilated ICU patients.
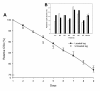
Methods: Muscle gene/protein expression, post-translational modifications (PTMs), muscle membrane excitability, muscle mass measurements, and contractile properties at the single muscle fiber level were explored in seven deeply sedated and mechanically ventilated ICU patients (not exposed to systemic corticosteroid hormone treatment, post-synaptic neuromuscular blockade or sepsis) subjected to unilateral passive mechanical loading for 10 hours per day (2.5 hours, four times) for 9 ± 1 days.
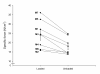
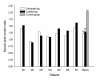
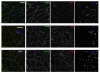
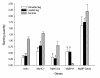
Results: These patients developed a phenotype considered pathognomonic of CIM; that is, severe muscle wasting and a preferential myosin loss (P < 0.001). In addition, myosin PTMs specific to the ICU condition were observed in parallel with an increased sarcolemmal expression and cytoplasmic translocation of neuronal nitric oxide synthase. Passive mechanical loading for 9 ± 1 days resulted in a 35% higher specific force (P < 0.001) compared with the unloaded leg, although it was not sufficient to prevent the loss of muscle mass.
Conclusion: Mechanical silencing is suggested to be a primary mechanism underlying CIM; that is, triggering the myosin loss, muscle wasting and myosin PTMs. The higher neuronal nitric oxide synthase expression found in the ICU patients and its cytoplasmic translocation are forwarded as a probable mechanism underlying these modifications. The positive effect of passive loading on muscle fiber function strongly supports the importance of early physical therapy and mobilization in deeply sedated and mechanically ventilated ICU patients.
Figures

References
-
- Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, Al-Saidi F, Cooper AB, Cook D, Slutsky AS, Herridge MS. Two-year outcomes, health care use and costs in survivors of ARDS. Am J Respir Crit Care Med. 2006;16:538–544. - PubMed
-
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;16:683–693. doi: 10.1056/NEJMoa022450. - DOI - PubMed
-
- Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;16:1293–1304. doi: 10.1056/NEJMoa1011802. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

