Crossover inhibition as an indicator of convergent evolution of enzyme mechanisms: a β-lactamase and a N-terminal nucleophile hydrolase
- PMID: 23098756
- PMCID: PMC3505995
- DOI: 10.1016/j.febslet.2012.10.019
Crossover inhibition as an indicator of convergent evolution of enzyme mechanisms: a β-lactamase and a N-terminal nucleophile hydrolase
Abstract
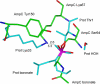
O-Aryloxycarbonyl hydroxamates and 1,3,4-oxathiazol-2-ones have been identified as covalent inhibitors of β-lactamases and proteasomes, respectively. The products of these inhibition reactions are remarkably similar, involving carbonyl cross-linking of the active sites. We have cross-checked these inhibitors, showing that the former inhibit proteasomes and the latter β-lactamases, to form the same inactive carbonyl adducts. These results are discussed in terms of similarities of the active site structures and catalytic mechanisms. It is likely that a mechanistic imperative has led to convergent evolution of these enzyme active sites, of a β-lactam-recognizing enzyme and a N-terminal protease belonging to different amidohydrolase superfamilies.
Copyright © 2012 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


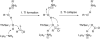

References
-
- Frére J-M, editor. Beta-Lactamases. Nova, NY: 2012.
-
- Wyrembak PN, Babaoglu K, Pelto RB, Shoichet BK, Pratt RF. O-Aryloxycarbonyl hydroxamates: New ß-lactamase inhibitors that cross-link the active site. J. Am. Chem. Soc. 2007;129:9548–9549. - PubMed
-
- Borissenko L, Groll M. 20S Proteasome and its inhibitors: Crystallographic knowledge for drug development. Chem. Rev. 2007;107:687–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

