Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial
- PMID: 23099009
- PMCID: PMC5380388
- DOI: 10.1016/S1470-2045(12)70414-X
Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial
Abstract
Background: Plexiform neurofibromas are slow-growing chemoradiotherapy-resistant tumours arising in patients with neurofibromatosis type 1 (NF1). Currently, there are no viable therapeutic options for patients with plexiform neurofibromas that cannot be surgically removed because of their proximity to vital body structures. We undertook an open-label phase 2 trial to test whether treatment with imatinib mesylate can decrease the volume burden of clinically significant plexiform neurofibromas in patients with NF1.
Methods: Eligible patients had to be aged 3-65 years, and to have NF1 and a clinically significant plexiform neurofibroma. Patients were treated with daily oral imatinib mesylate at 220 mg/m(2) twice a day for children and 400 mg twice a day for adults for 6 months. The primary endpoint was a 20% or more reduction in plexiform size by sequential volumetric MRI imaging. Clinical data were analysed on an intention-to-treat basis; a secondary analysis was also done for those patients able to take imatinib mesylate for 6 months. This trial is registered with ClinicalTrials.gov, number NCT01673009.
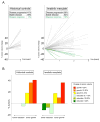
Findings: Six of 36 patients (17%, 95% CI 6-33), enrolled on an intention-to-treat basis, had an objective response to imatinib mesylate, with a 20% or more decrease in tumour volume. Of the 23 patients who received imatinib mesylate for at least 6 months, six (26%, 95% CI 10-48) had a 20% or more decrease in volume of one or more plexiform tumours. The most common adverse events were skin rash (five patients) and oedema with weight gain (six). More serious adverse events included reversible grade 3 neutropenia (two), grade 4 hyperglycaemia (one), and grade 4 increases in aminotransferase concentrations (one).
Interpretation: Imatinib mesylate could be used to treat plexiform neurofibromas in patients with NF1. A multi-institutional clinical trial is warranted to confirm these results.
Funding: Novartis Pharmaceuticals, the Indiana University Simon Cancer Centre, and the Indiana University Herman B Wells Center for Pediatric Research.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no financial or personal interests that would inappropriately influence their work on this manuscript.
Figures

Comment in
-
Treatment for plexiform neurofibromas in patients with NF1.Lancet Oncol. 2012 Dec;13(12):1175-6. doi: 10.1016/S1470-2045(12)70435-7. Epub 2012 Oct 23. Lancet Oncol. 2012. PMID: 23099008 No abstract available.
References
-
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–33. - PubMed
-
- Wolkenstein P, Rodriguez D, Ferkal S, et al. Impact of neurofibromatosis 1 upon quality of life in childhood: a cross-sectional study of 79 cases. Br J Dermatol. 2009;160:844–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

